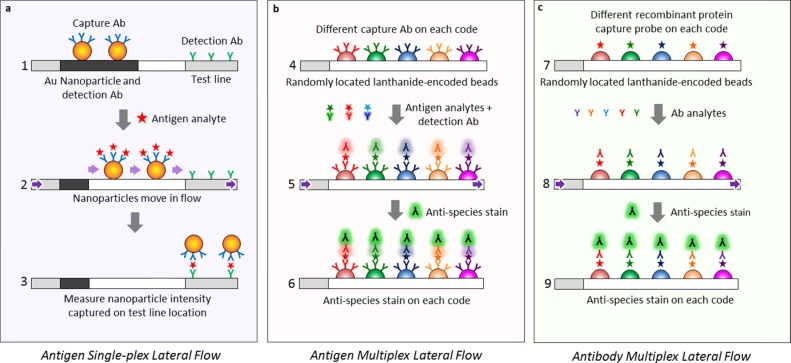
Figure 1.
Comparison between (a) traditional single-plex lateral flow assay, where the analyte is identified by localization onto a specific location (the capture line), and the new (b, c) MFAs where an analyte is identified via its optical code rather than onto a specific geometric location. A traditional lateral flow assay has a detection Ab attached to an Au nanoparticle and a capture Ab bound to the test line at a specific location (a1). The antigen within the analyte solution moves through the flow medium (purple arrows), bonds to the capture Ab (a2), and the tripartite antigen/capture Ab/Au nanoparticle moiety localizes onto a second Ab at the test line location (a3), indicating a positive antigen test. In contrast, the MFA assay for antigens (b) or antibodies (c) instead of nanoparticles uses a multitude of lanthanide-encoded beads contacting the flow medium to identify the captured analyte, and only molecular species move within the flow. To identify Ags using sandwich ELISA in a multiplexed fashion (b), the beads are derivatized with capture probe Abs, with each capture probe bound to a bead set with a unique optical code (b4), exposed to the Ag analyte solution, treated with the detection Ab (b5), and stained with antispecies stain or streptavidin–phycoerythrin (SAPE) to measure the amount of Ag (b6). To identify Abs instead of Ags in a multiplexed assay (c), a recombinant protein, which acts as an Ag against the Ab of interest, is attached to the lanthanide-encoded bead set (c7), exposed to the Ab analyte solution (c8), and labeled with an antispecies stain against the captured Ab (c9) to give the amount of analyte.