Abstract
Chronic inflammation is a major cancer predisposition factor. Constitutive activation of the inflammation-driving NF-κB pathway commonly observed in cancer or developed in normal tissues because of persistent infections or endogenous tissue irritating factors, including products of secretion by senescent cells accumulating with age, markedly represses p53 functions. In its turn, p53 acts as a suppressor of inflammation helping to keep it within safe limits. The antagonistic relationship between p53 and NF-κB is controlled by multiple mechanisms and reflects cardinal differences in organismal responses to intrinsic and extrinsic cell stresses driven by these two transcription factors, respectively. This provides an opportunity for developing drugs to treat diseases associated with inappropriate activity of either p53 or NF-κB through targeting the opposing pathway. Several drug candidates of this kind are currently in clinical testing. These include anticancer small molecules capable of simultaneous suppression of p53 and activation of NF-κB and NF-κB-activating biologics that counteract p53-mediated pathologies associated with systemic genotoxic stresses such as acute radiation syndrome and side effects of cancer treatment.
Prolonged NF-κB activation can promote inflammation, suppress p53 activity, and increase the risk of cancer. Drugs that modulate the NF-κB or p53 pathways by targeting the opposing pathway may have therapeutic value.
INFLAMMATION: DEFINITION AND BIOLOGICAL SIGNIFICANCE
Inflammation is generally regarded as an adaptive response to infection, tissue injury, and other harmful stimuli and agents that includes a wide variety of physiological and pathological processes geared toward limiting their adverse effects (Goto 2008; Medzhitov 2008). However, it is becoming increasingly recognized that, when chronic, inflammation can in itself be dangerous, particularly in terms of cancer risk. This review describes work supporting the connection between chronic inflammation and tumor development and progression and the impact of mutual regulation between the major inflammation regulator NF-κB and the major tumor suppressor p53 on the balance of these processes.
Inflammatory responses are triggered through activation of germline-encoded pattern-recognition receptors (PRRs) expressed in both immune and nonimmune cells by microbial structures known as pathogen-associated molecular patterns (PAMPs). Some PRRs are also able to sense various endogenous (nonmicrobial) signals that arise during tissue or cell damage and are commonly referred to as danger-associated molecular patterns (DAMPs) (Medzhitov 2008).
Acute inflammation is usually caused by exogenous stimuli including, besides infection and injury, allergens, irritants, foreign bodies, and toxic compounds. A successful acute inflammatory response results in elimination of the infectious agent (or other stimulus) followed by resolution of inflammation and repair of tissue damage. If the acute inflammatory response fails to eliminate the pathogen or other stimulus, the inflammatory process may become chronic (Drayton et al. 2006).
Chronic inflammation (also referred to as “para-inflammation”; Medzhitov 2008) shares many features of acute inflammation, but is usually low grade and persistent. Although acute inflammation serves to protect and promote regeneration of at-risk tissue, chronic inflammation is typically associated with tissue degeneration (Franceschi et al. 2000b; Franceschi and Campisi 2014). In terms of inducing stimuli, unlike acute inflammation, chronic inflammation is often caused by endogenous inducers produced by stressed, damaged, or malfunctioning tissues (Medzhitov 2008) rather than exogenous inducers. For example, certain cellular constituents released during necrotic cell death, including ATP, K+ ions, HMGB1 (high-mobility group box 1 protein), several members of the S100 calcium-binding protein family, crystals of monosodium urate, and reactive oxygen species (ROS), play a role in chronic inflammation (Navab et al. 2006; Bianchi 2007; Rock and Kono 2008). Damaged mitochondria release another set of DAMPs (formyl peptides, mitochondrial DNA, cardiolipin) that are powerful activators of inflammation because of their evolutionarily conserved similarities to bacterial PAMPs (Zhang et al. 2010; Iyer et al. 2013).
On the molecular level, the key regulator of both acute and chronic inflammation is the NF-κB signaling pathway controlled by its central transcription factor, NF-κB (Ben-Neriah and Karin 2011). PRR signaling induced by PAMPs and DAMPs proceeds through a cascade of biochemical events resulting in release of NF-κB from its cytoplasmic inhibitor IkBa. Once released from IkBa, NF-κB translocates into the nucleus where it activates transcription of numerous target genes whose products create the physiological manifestations of inflammation. NF-κB activation typically involves up-regulation of antiapoptotic factors (e.g., c-FLIP, Bcl-XL, A1/Bfl-1, c-IAPs, XIAP, TRAF1, TRAF2) and antioxidants (e.g., MnSOD, ferritin heavy chain, glutathione S-transferase, metallothionein) that serve to increase the resistance of cells to stresses (cell-intrinsic responses) (Luo et al. 2005).
NF-κB signaling was shown to control both cell proliferation (La Rosa et al. 1994; Guttridge et al. 1999) and apoptosis (Perkins 1997). Extrinsic effects of NF-κB activation include increased production of endogenous antibacterial factors, numerous cytokines and chemokines involved in generating immune responses, and adhesion molecules that mediate recruitment of leukocytes to sites of inflammation (Karin 2009).
The NF-κB activation response is strong, short lasting, and reversible under conditions of acute inflammation. The NF-κB response is shut down via a negative feedback loop mechanism involving induction of the NF-κB inhibitor, IkBa, which is encoded by a NF-κB-responsive gene (Chiao et al. 1994; Ak and Levine 2010). In contrast, with chronic inflammation, NF-κB activation is modest but long lasting. This may be because of insufficient expression of IkBa or reactivation of NF-κB by constitutively present PAMPs or DAMPs resulting in long-term oscillation of NF-κB pathway activity (Chiao et al. 1994; Ak and Levine 2010).
Another inflammatory pathway that is not triggered by NF-κB but is still NF-κB dependent at its downstream stages is driven by type I interferons (IFNs) including IFN-α and IFN-β. This is a widely expressed family of effector cytokines that promotes innate antiviral and antibacterial immunity (Davidson et al. 2015). Microbial gene products such as dsRNA, ssRNA, dsDNA, ssDNA, or cell-wall constituents (PAMPs) bind to specific TLRs (Toll-like receptors) to trigger type I IFN synthesis (Gonzalez-Navajas et al. 2012). Engagement of IFN receptors up-regulates the JAK/STAT pathways in which the transcription factor ISGF3 triggers transcription of IFN-stimulated genes (ISGs) leading to an inflammatory response (Davidson et al. 2015). Coupled with induction of antiviral proteins, type I IFN also induces secretion of cytokines and chemokines that promote inflammatory responses and pathways important for clearance of infected cells. Moderate type I IFN responses to infection are protective, whereas excessive amounts of IFNs can contribute to immunopathology (Davidson et al. 2015). In contrast to “emergency” production of IFNs induced by virus or bacterial infection, “physiological” low levels of IFNs may be produced continuously and may thus maintain physiological functions, such as homeostasis of immune cell activities and resistance to cancer (Gonzalez-Navajas et al. 2012; Davidson et al. 2015). Aberrant production of endogenous IFNs can also contribute to immunopathologies such as autoimmune and inflammatory disorders (e.g., inflammatory bowel disease, systemic lupus erythematosus, psoriasis, and multiple sclerosis) (Cheon et al. 2014). Increased endogenous IFN production can also have procancer effects through induction of DNA damage–resistant gene products in response to persistent infection by DNA and RNA viruses or exposure to DNA damaging agents such as UV light or DNA damaging chemicals (Cheon et al. 2014). Furthermore, the majority of malignant tumors have constitutively active NF-κB (and therefore should be considered as having chronic inflammation), and their viability frequently depends on this property (Karin 2006). Many of the signaling pathways implicated in cancer are likely networked to the activation of NF-κB (Karin 2009; Grivennikov et al. 2010).
CHRONIC INFLAMMATION AS A MAJOR CANCER PREDISPOSITION FACTOR
Numerous correlations between chronic inflammation and cancer have led to a well-accepted paradigm of a causative relationship between the two (recently reviewed in Gudkov et al. 2011). Conditions of chronic inflammation leading to cancer may be attributed to infectious pathogens such as Helicobacter pylori, which is associated with gastric cancer and MALT-lymphoma (Loffeld et al. 1990; Stolte 1992), Schistosoma haematobium, which is associated with bladder cancer (Gelfand et al. 1967), and Opisthorchis viverrini, which is associated with cholangiocarcinoma (Schwartz 1980). Persistent viral infection is thought to be a major cause of hepatocellular carcinoma (HCC). About 90% of HCC develops because of chronic infection by various agents such as hepatitis B and hepatitis C viruses (Sherlock et al. 1970; Kiyosawa et al. 1984; Chang 2007; Rook and Dalgleish 2011). It was found that some viruses, such as Epstein–Barr virus (EBV), human herpesvirus 8 (HHV8), human T-lymphotropic virus 1 (HTLV-1), and human papilloma virus (HPV), can promote transformation of epithelial cells by transducing dominant oncogenes, possibly without involving inflammation. EBV is conditionally responsible for several cancers such as Hodgkin lymphoma, Burkitt lymphoma, nasopharyngeal carcinoma, and lymphoma in the central nervous system (CNS) (James et al. 1997; Coussens and Werb 2002; Kutok and Wang 2006). Degradation of p53 by HPV protein E6 may be an important factor in the development of cervical cancer (Scheffner et al. 1990). Chronic inflammation resulting from environmental factors, such as exposure to asbestos, smoke, and UV irradiation, are associated with lung and skin cancer (Mossman et al. 2006; Vaid and Katiyar 2010). Support for the notion of inflammation as a cancer predisposing factor is also provided by examples of cancer development from pathologies that involve noninfectious inflammatory conditions induced by imbalanced immune regulation such as Barrett’s esophagitis, chronic pancreatitis, inflammatory bowel diseases (ulcerative colitis and Crohn’s disease), or age-related chronic inflammation of prostate tissue associated with secretion of proinflammatory factors by senescent cells (Goh et al. 2006; Karin 2006; Levine and Oren 2009; Lane and Levine 2010; Goldstein et al. 2011; Gudkov et al. 2011). Finally, numerous clinical studies have revealed a strong correlation between chronic inflammation and tumor formation (Coussens and Werb 2002; Balkwill and Coussens 2004; de Visser et al. 2006; Lin and Karin 2007), leading to estimations that >15% of cancer cases might be caused by chronic inflammation (Kuper et al. 2000; Sunami and Wirth 2011). Hence, chronic inflammation is a powerful risk factor for cancer development with an overall impact on cancer incidence exceeding that of genetic predispositions (based on National Cancer Institute data, inherited mutations are thought to play a role in ∼5%–10% of all cancers).
INFLAMMATION CAN SUPPRESS p53 FUNCTION
The mechanisms involved in translation of chronic inflammation into cancer remain largely hypothetical. One hypothesis is that changes in the microenvironment associated with chronic inflammation might enable unconstrained growth of cells. The reversibility of primary gastric adenocarcinomas following eradication of H. pylori infection is an illustration of this possibility (Graham 2015). The polyclonal nature of primary prostate cancer in elderly men suggests that early stage prostate cancer developing on the background of a chronically inflamed organ is another example of this type of case (Logunov et al. 2008). Another possible mechanism is that activation of inflammation-driving pathways in cells that are under constant influence of PAMPs and/or DAMPs can interfere with the activity of tumor-suppressor mechanisms, ultimately enabling accumulation of individual epigenetic and genetic changes that can cause cancer. Antiapoptotic factors expressed by viruses, including those known to be carcinogenic, illustrate this type of mechanism (Thompson and Kurzrock 2004; Andoniou and Degli-Esposti 2006). Regardless of the underlying mechanism(s), however, the link between inflammation and cancer suggests that inflammation, largely controlled by NF-κB, must interfere with natural tumor-suppressor mechanisms, of which p53 is the most important. Hence, an obvious hypothesis is that the carcinogenicity of chronic inflammation is due to p53 suppression by activated NF-κB.
The first support for this idea was provided by our finding that the inactivation or attenuation of p53 function that is observed in cancers that retain wild-type p53 expression can be imposed by constitutively active NF-κB. Genetic or pharmacological inhibition of constitutively active NF-κB in a variety of tumor-derived cell lines resulted in activation of p53 function and tumor cell death via p53-dependent apoptosis (Gurova et al. 2005). These findings suggest that tumors that acquire activation of NF-κB do not benefit further from p53 mutations because wild-type p53 activity is already “eliminated” through the activity of NF-κB, which creates conditions phenotypically equivalent to genetic p53 deficiency. This phenomenon provided the rationale for development of curaxins, anticancer drug candidates that are capable of simultaneously suppressing NF-κB and activating p53 (Gasparian et al. 2011) and are currently in clinical trials (clinicaltrials.gov/ct2/show/NCT01905228).
This conclusion is supported by a series of observations indicating that the effects of constitutively active NF-κB in cell transformation models in vitro are similar to those caused by p53 suppression. One of the well-characterized tumor-suppressor functions of p53 is its ARF-mediated response to the activation of dominant oncogenes resulting in establishment of premature senescence (Evan and d’Adda di Fagagna 2009). If NF-κB activation leads to sufficient suppression of p53 activity, one would expect that under conditions of constitutively active NF-κB cell transformation via oncogenic Ras could occur even in the presence of wild-type p53 expression. Our observations indicate that mycoplasma infection (as a constitutive extrinsic NF-κB activating agent) plays the role of a p53-suppressing oncogene that cooperates with Ras in cell transformation and suggest that the carcinogenic and mutagenic effects of mycoplasma might be because of NF-κB-mediated inhibition of p53 (Logunov et al. 2008; Barykova et al. 2011). A clinical study showed that micoplasm (Mycoplasma hominis) was present three times more frequently in patients with prostate cancer than in those with benign prostatic hyperplasia (Barykova et al. 2011). In addition, prostate cancer–positive men had higher titers of antibodies against M. hominis, and average PSA levels were higher in M. hominis–positive men. These data, together with previous observations linking mycoplasma infection with cell transformation, genomic instability, and resistance to apoptosis, suggest that M. hominis infection may be involved in development of prostate cancer and may, therefore, be a potential diagnostic marker and/or target for improved prevention and treatment of this disease.
The effects of live mycoplasma were partially mimicked in vitro by a lipopeptide component of the mycoplasma surface, the TLR2 agonist R-Pam2. Supplementation of the cell-culture medium with R-Pam2 resulted in a substantial delay in the onset of replicative senescence in mouse embryo fibroblasts, which is known to be regulated by p53 (D Logunov, V Natarajan, and AV Gudkov, unpubl.).
Sufficiency of NF-κB activation for overcoming p53’s ability to trigger oncogene-induced senescence was shown in our experiments using a set of NF-κB-activating selectable peptides (NASPs) (Natarajan et al. 2014). Transduction of NASPs into mouse and rat embryo fibroblasts did not, in itself, alter their growth. However, when coexpressed with oncogenic Ras (H-RasV12), NASPs allowed rodent fibroblasts to overcome H-RasV12-mediated, p53-dependent senescence and acquire a transformed tumorigenic phenotype. Consistent with their ability to cooperate with oncogenic Ras in cell transformation, NASP expression reduced the transactivation activity of p53. This experimental system provides an in vitro model of NF-κB-driven carcinogenesis and suggests that the known carcinogenic effects of inflammation may be at least partially because of NF-κB-mediated abrogation of oncogene-induced senescence.
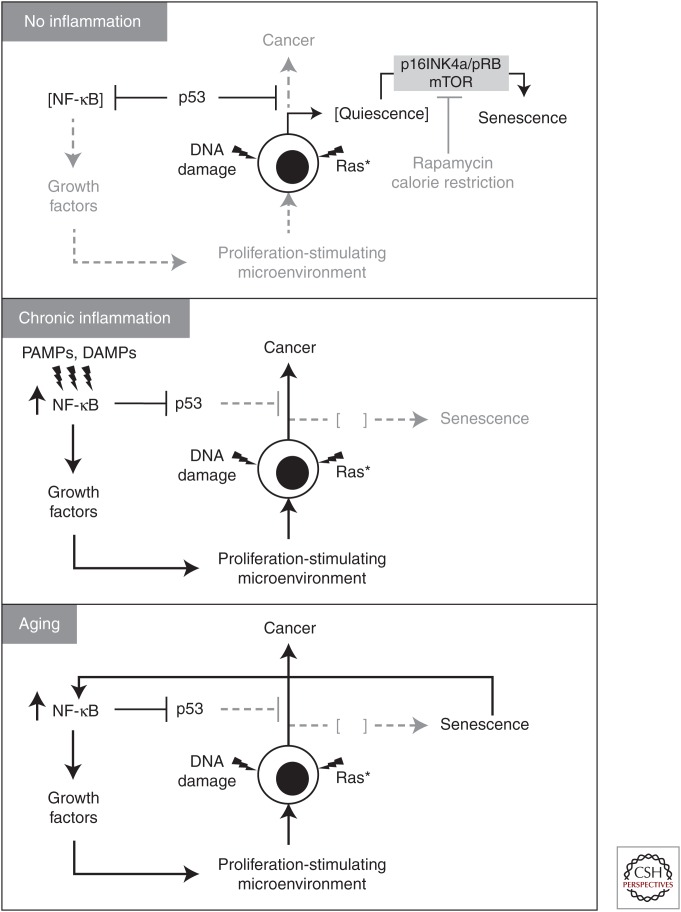
As described above, three different means of mimicking the tumor-specific property of constitutive NF-κB activation–(1) chronic infection (mycoplasma), (2) a pharmacological agent (TLR2 agonist), and (3) NF-κB-activating peptides—all show attenuation of p53 function and establishment of permissive conditions for transformation. These results clearly show that NF-κB activation and establishment of inflammation create a situation in which p53 can no longer effectively exert its function as an eradicator of transformation-prone cells (Fig. 1). However, despite the association between loss of p53 function and cancer, short-term NF-κB-mediated suppression of p53 is not carcinogenic. Numerous concerns were originally raised about the safety of p53-suppressive drugs, but it is now clear that short-term, reversible suppression of p53 does not translate into an increased risk of cancer development (Komarov et al. 1999; Christophorou et al. 2005, 2006). The fact that short-term NF-κB induction is not carcinogenic is supported by the absence of any link between the occurrence of diseases associated with acute inflammation and cancer. Chronic inflammation, however, is a completely different story because it exposes cells in inflamed tissues to a p53-deficient environment for lengths of time sufficient for the acquisition of genetic and epigenetic alterations supporting advanced transformed phenotypes. This makes chronic inflammation a dangerous pathophysiological condition that is functionally equivalent to a p53-suppressing oncogene.
Figure 1.
Models describing potential cellular mechanisms linking chronic inflammation and cancer development. See explanations in the text.
p53 CAN SUPPRESS INFLAMMATION
The relationship between NF-κB and p53 is not one of unidirectional regulation but rather reciprocal regulation. There are a number of studies indicating that p53 is a negative regulator of inflammation. First, a significant proportion of tumor-prone p53-null mice (25%) die before tumor development from unresolved inflammation that results in abscesses, gastroenteritis, or myocarditis, suggesting that innate immune responses are deregulated in these mice (Donehower et al. 1992). In addition, manifestations of autoimmune diseases, including collagen-induced arthritis, and experimental autoimmune encephalitis and diabetes, were found to be more severe in p53-deficient mice than in wild-type mice (Yamanishi et al. 2002; Okuda et al. 2003; Zheng et al. 2005; Park et al. 2013). Park et al. (2013) showed that p53 acts as a negative regulator of autoimmunity, not only by modifying the immunological milieu via suppressing inflammatory cytokines, but also by directly controlling T-cell differentiation. These data are consistent with a recent observation that mice lacking p53, especially in combination with inactivation of the p53 downstream target, p21, spontaneously develop autoimmunity (Salvador et al. 2002, 2005).
Accelerated growth of atherosclerotic plaques was observed in p53–/–/apoE–/– mice as compared with p53+/+apoE–/– mice and in low-density lipoprotein (LDL) receptor-knockout mice that were lethally irradiated and transplanted with bone marrow from p53–/– mice as compared with the same mice transplanted with p53 wild-type bone marrow (Guevara et al. 1999; van Vlijmen et al. 2001; von der Thusen et al. 2002; Merched et al. 2003). The observed acceleration in plaque growth was associated with increased invasion of activated macrophages into the plaques. Similarly, ionizing radiation was shown to induce faster and stronger invasion of inflammatory cells and fibroblasts into damaged tissues in p53-null mice than in wild-type mice (Komarova et al. 2004). Specific ablation of the p53 gene in mouse epidermis led to spontaneous development of aggressive squamous cell carcinoma preceded by inflammation (Martinez-Cruz et al. 2009). In addition, inflammatory infiltration of the lung and subsequent disruption of alveolar architecture caused by chronic exposure to the DNA-damaging agent bleomycin was markedly increased in p53-null mice and transgenic mice expressing mutant p53 in the lung as compared with wild-type mice (Davis et al. 2000; Ghosh et al. 2002). We also observed more severe inflammatory responses and accelerated development of fibrosis in the lung of p53–/– mice as compared with wild-type mice after γ-irradiation (EA Komarova and AV Gudkov, unpubl.).
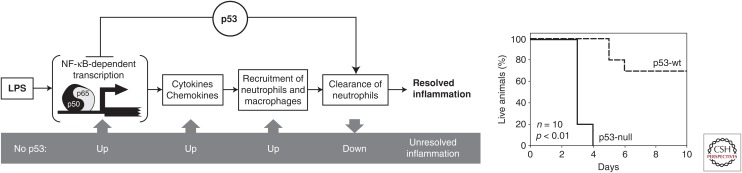
Consistent with the observations described above, we found that p53 inhibits inflammation by acting as an antagonist of NF-κB (Fig. 2) (Komarova et al. 2005). This was first suggested by our observation of striking similarities in the global gene expression profiles of human LNCaP prostate cancer cells transduced with a p53-inhibitory genetic element or treated with tumor necrosis factor (TNF). This data suggested that p53 inhibits transcription of TNF-inducible genes, many of which are known to be regulated by NF-κB (TNF is a known activator of NF-κB). In support of this, ectopically expressed p53 was shown to inhibit transcription from NF-κB-dependent promoters. Furthermore, suppression of inflammatory responses by p53 was observed in vivo at the molecular level (reduced transcription of genes encoding cytokines and chemokines and reduced accumulation of ROS in p53-null mice), the cellular level (activation of macrophages and neutrophils and their hypersensitivity to lipopolysaccharide [LPS] in p53-null mice), and the organismal level (high levels of metabolic markers of inflammation in tissues of p53-null mice). Later, similar results were described by Liu et al. (2009) in a mouse model of LPS-induced lung injury. Enhanced induction of NF-κB-dependent cytokines was detected in the infected p53–/– mouse lung. Also, infected p53–/– mice showed increased mortality associated with aggravated lung injury (Madenspacher et al. 2013). Interestingly, p21-null mice have an overactivated inflammatory phenotype that is similar to that of p53-null mice. This phenotype included increased susceptibility to endotoxic shock and elevated serum levels of cytokines (Scatizzi et al. 2009; Trakala et al. 2009).
Figure 2.
Increased inflammatory response in p53-null mice (scheme of p53-mediated regulation of inflammation). p53 acts at least in two stages of inflammation being a general inhibitor of NF-κB-dependent transcription and a positive regulator of neutrophil clearance by macrophages. Lack of p53 results in overreaction to proinflammatory stimuli (lipopolysaccharide [LPS]) and hypersensitivity of p53-null mice (death). wt, wild type. (From Komarova et al. 2005; with permission, from the authors.)
It is interesting that mice with a P72R mutation (substitution of proline 72 with arginine) in their p53 gene have a markedly enhanced response to inflammatory challenges, whereas, in humans, codon-72 polymorphism is associated with a cancer-prone phenotype (Frank et al. 2011). Also, mice that express hypomorphic R172P mutant p53 (a mutation that abrogates p53-mediated apoptosis while keeping cell-cycle control intact) are more sensitive to ultraviolet light–induced skin inflammation than wild-type mice (Tavana et al. 2010).
It was shown that p53 can regulate polarization of macrophages in the M1 direction, which is characterized by the expression of a wide range of proinflammatory genes (Zheng et al. 2005; Liu et al. 2009). Recently, it was shown that p53 activity was also increased when macrophages were polarized to the M2 subtype, which is characterized by expression of high levels of anti-inflammatory and tissue repair marker genes (Li et al. 2014). p53 activation led to reduced expression of M2 genes. In contrast, increased expression of M2 genes was apparent in M2-polarized macrophages from p53-deficient and p53 mutant mice.
The most frequent p53 mutations observed in human cancers are single amino acid substitutions. These mutant p53 proteins often reach high levels in cells because they are more stable than the wild-type protein and may lead to a cancer-promoting gain of function (Sigal and Rotter 2000; Cadwell and Zambetti 2001). This is supported by the finding that mutant p53 “knock-in” mice displayed a broader tumor spectrum and increased aggressiveness and metastatic potential than wild-type mice (Lang et al. 2004; Olive et al. 2004). Mutant p53 gain-of-function may involve inhibition of cell death, increased genomic instability, enhanced cell invasion, and abnormal metabolism (Oren and Rotter 2010; Muller and Vousden 2013). Recently, it was reported that expression of various p53 mutants correlated with increased NF-κB expression in cultured cells and in mouse models (Scian et al. 2005; Gulati et al. 2006; Weisz et al. 2007). Analysis of human head and neck tumors and lung tumors revealed a close correlation between the presence of abundant mutant p53 proteins and constitutive activation of NF-κB. Also, elevated expression of NF-κB-dependent chemokines, such as CXCL5, CXCL8, and CXCL12, was found in cells expressing several p53 mutants (Yeudall et al. 2012). Mutant p53 enhanced activation of NF-κB by TNF, and inhibition of endogenous mutant p53 sensitized cancer cells to cytokine-induced death. It is important that elevated mutant p53 protein correlated with increased NF-κB activation in human premalignant and malignant lesions (Cooks et al. 2014). These findings suggest a role for cooperation of mutant p53 and NF-κB in development of cancer under conditions of chronic inflammation such as exposure to inflammatory cytokines. One mechanism that appears to contribute to this is that mutant p53 can facilitate transcriptional activation of NF-κB by promoting binding of p65 to κB sites within chromatin (Schneider et al. 2010; Cooks et al. 2013).
One well-documented link between chronic inflammation and human cancer involves colitis-associated colorectal cancer (Asquith and Powrie 2010; Ullman and Itzkowitz 2011). In contrast to sporadic colorectal cancer in which p53 mutations play a tumor-promoting role, in the colitis-associated form of the disease, p53 mutations are often observed very early, suggesting that they play a tumor-initiating role (Hussain et al. 2000; Ullman and Itzkowitz 2011). Thus, the role of p53 mutations in driving colorectal cancer may differ depending on the inflammatory microenviroment. Cooks et al. (2013) showed that mutant p53 (p53R273H) prolonged TNF-α-induced NF-κB activation in cultured cells and intestinal organoid cultures, suggesting that specific mutations in p53 not only abolished the anti-inflammatory properties of wild-type p53 but also actively intensified and prolonged the inflammatory response. Exposure of mice harboring a germline G515A p53 mutation (Lang et al. 2004) to dextran sulfate sodium (DSS) resulted in development of severe chronic inflammation and persistent tissue damage, and greatly increased the incidence of inflammation-associated colon cancer. This p53 gain-of-function mutation led to rapid onset of flat dysplastic lesions that progressed to invasive carcinoma with mutant p53 accumulation and augmented NF-κB activation, faithfully recapitulating features frequently observed in human colitis-associated colorectal cancer.
The majority of observations described above indicate that p53 can act as an attenuator of inflammation in vivo through its ability to suppress the activity of NF-κB. This role is consistent with both the tumor-suppressor function of p53 (because chronic inflammation is frequently associated with tumorigenesis) and the constitutive activation of NF-κB that is commonly observed in tumors, the majority of which are p53-deficient.
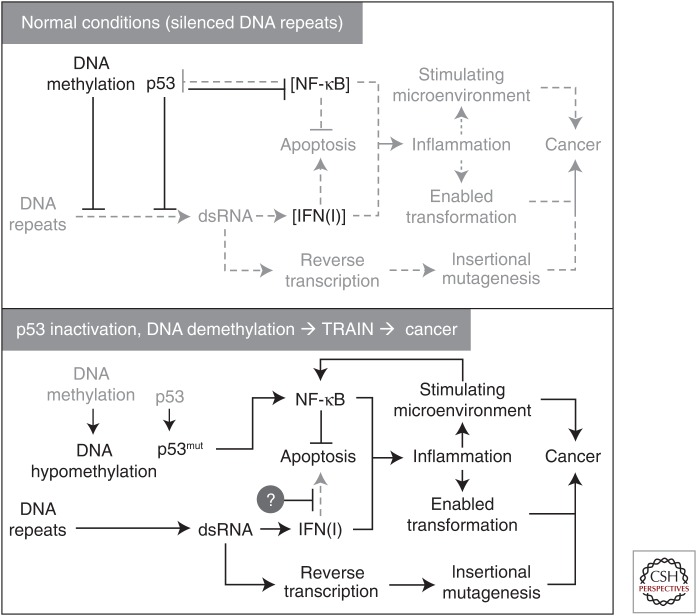
Recently, another anti-inflammatory function of p53 that is independent on its antagonistic relationships with NF-κB was discovered. p53 was found to play a major role, along with DNA methylation, in epigenetic transcriptional silencing of repetitive DNA retroelements and certain classes of satellite DNA (Leonova et al. 2013). DNA hypomethylation in the absence of p53 results in massive transcription of these normally silent classes of repeats, leading to generation of large amounts of double-stranded RNA, which activates a suicidal type I IFN response (a phenomenon named “transcription of repeats activates IFN” [TRAIN]) (Fig. 3). Tumors with impaired p53 function frequently acquire constitutively active but nonfunctional IFN signaling. This newly identified p53 function could contribute to its tumor-suppressor activity because it protects cells from the risk of insertional mutagenesis associated with derepression of retrotransposons (Leonova et al. 2013). These observations allow p53 to be defined as a repressor of IFN-mediated inflammation driven by expression of virus-like RNA species.
Figure 3.
Scheme of regulation of epigenetic transcriptional silencing of DNA repeats and inflammatory consequences of its deregulation by hypomethylation of DNA in the cells with impaired p53 function (Leonova et al. 2013). dsRNA, double-stranded RNA; IFN, interferon.
MOLECULAR MECHANISMS OF MUTUAL NEGATIVE REGULATION OF p53 AND NF-κB
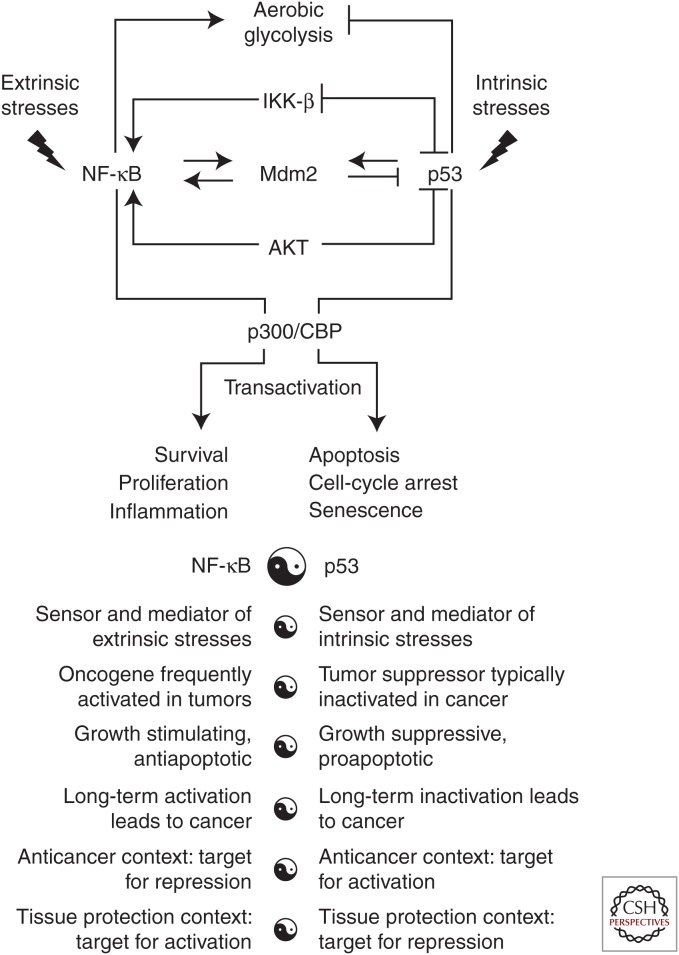
Given the generally opposite nature of the responses initiated by p53 and NF-κB, there is a clear physiological need to coordinately regulate their relative activities to best serve the organism. Cross talk between the p53 and NF-κB pathways occurs on multiple levels and the results of this cross talk depend on the specific cellular context and stress stimulus (Fig. 4) (Schneider and Kramer 2011). Cooperation (rather than the expected antagonism) of p53 and NF-κB has been reported in some cell systems. For example, under some specific conditions, the typically prosurvival factor NF-κB can actually promote cell death (Campbell et al. 2004; Janssens and Tschopp 2006; Strozyk et al. 2006) and proapoptotic p53 can promote cell survival (Lassus et al. 1996; Ryan et al. 2000; Janicke et al. 2008). It was shown that in response to double-strand DNA breaks p53 and NF-κB were coordinately activated and contributed together to the resultant changes in gene expression (Elkon et al. 2005). Cross talk between p53 and NF-κB might also be necessary for full activation of NF-κB in response to certain types of stimuli such as TNF-α (Schneider and Kramer 2011). This positive cooperation with NF-κB seems to be stronger for p53 mutants than the wild-type protein, providing a potential explanation for the fact that p53 mutants are much more frequently observed in cancer than p53 deletions (Schneider et al. 2010; Hoesel and Schmid 2013). p53 and NF-κB also cooperated in the induction of oncogene-induced senescence, in which NF-κB promoted clearance of oncogene-expressing cells by the immune system (Chien et al. 2011; Hoesel and Schmid 2013). However, as described in the preceding sections, the more commonly observed relationship between NF-κB and p53 is antagonistic (Scian et al. 2005; Lee et al. 2007; Weisz et al. 2007; Szoltysek et al. 2008).
Figure 4.
Schematic model summarizing molecular mechanisms that underlie mutual negative regulation of p53 and NF-κB. Listed are the major properties of p53 and NF-κB showing opposite biological functions of these pathways. See explanations in the text. CBP, CREB-binding protein.
Despite substantial research efforts in this area, our understanding of the mechanisms that regulate p53/NF-κB cross talk remains limited. One possibility is that p53 and NF-κB repress the activities of each other through competition for limited pools of the histone acetyltransferase enzymes p300/CREB-binding protein (CBP) (Ravi et al. 1998; Wadgaonkar et al. 1999; Webster and Perkins 1999; Ikeda et al. 2000). This possibility is supported by the demonstration that both p53 and NF-κB physically interact with CBP and require such interaction for maximal activity (Avantaggiati et al. 1997; Gerritsen et al. 1997). Next, the tumor suppressor ARF, which raises p53 levels and activity through inhibition of Mdm2 (Honda and Yasuda 1999), can block NF-κB activity through activation of the ATR/CHK–1 DNA damage signaling complex (Eymin et al. 2006). ATR/CHK-1-mediated phosphorylation of NF-κB inhibits its ability to transcribe genes (Rocha et al. 2005). p53 can also restrict activation of the IKK-β–NF-κB pathway through suppression of glycolysis (Kawauchi et al. 2008, 2009; Ak and Levine 2010). In contrast to NF-κB transcriptional activity that favors increased glucose uptake, p53 activity results in reduced glucose uptake (Kawauchi et al. 2008). It was shown that absence of p53 leads to up-regulated expression of the glucose transporter GLUT3 (Kawauchi et al. 2008). In contrast, activation of p53 has been linked to reduced expression of several glycolysis-regulating factors, including glucose transporters (Schwartzenberg-Bar-Yoseph et al. 2004). Knockin mice with the P72 variant in p53 gene displayed increased NF-κB-dependent inflammatory gene expression, with increased chromatin association of the p65 subunit of NF-κB and enhanced response to lipopolysaccharide challenge (Frank et al. 2011). Recent data indicates that mutant p53 elevates expression of p52 NF-κB by inducing acetylation of histones via recruitment of CBP and Stat2 on its promoter via CBP-mediated acetylation (Vaughan et al. 2012).
A number of mechanisms have been described by which NF-κB can shut down p53 activity and p53 responses (Scian et al. 2005; Lee et al. 2007; Weisz et al. 2007; Szoltysek et al. 2008). Among documented models explaining NF-κB-mediated negative regulation of p53 is NF-κB-dependent up-regulation of the levels of the major p53 inhibitor, Mdm2, which is an E3 ubiquitin ligase that directs p53 degradation (Tergaonkar et al. 2002; Egan et al. 2004; Kashatus et al. 2006). Conversely, in the absence of p53, Mdm2 directly increases p65 promoter activity (Gu et al. 2002). It is known that AKT-1 protein kinase is activated through phosphoinositide-3-kinase (PI3K) pathway signaling (Alessi et al. 1997; Franke et al. 1997; Yang et al. 2004). Engagement of the PI3K pathway leading to activation of AKT-1 favors NF-κB signaling while suppressing p53 signaling (Bai et al. 2009). This occurs in part through AKT-1-mediated phosphorylation of IKK protein, which increases its activity and activates NF-κB. The inhibitory effect of PI3K/AKT-1 signaling on p53 activity can involve AKT-1-mediated phosphorylation and inactivation of p53-regulated proapoptotic factors (Datta et al. 1997) as well as AKT-1-mediated phosphorylation of Mdm2 (Ogawara et al. 2002), which increases p53 degradation. Other studies showed that IKK-α-induced CBP phosphorylation switches CBP’s binding preference from p53 to NF-κB, which results in increased NF-κB-dependent transcription and decreased p53-dependent transcription (Webster and Perkins 1999; Huang et al. 2007; Tergaonkar and Perkins 2007). It is important to note that IKK-α was also found to negatively regulate p53 function through an alternative mechanism involving NF-κB subunit p52 (Schumm et al. 2006; Shen and Tergaonkar 2009). It is known that the IκB kinase (IKK) complex activates NF-κB by phosphorylating its inhibitor IκB (Cooks et al. 2014). Also, IKK-β, a major component of the NF-κB pathway, can phosphorylate p53 leading to its ubiquitination and Mdm2-independent proteosomal degradation (Ikeda et al. 2000; Xia et al. 2009). Thus, it is possible that IKK-β, which is constitutively activated by extrinsic mediators of chronic inflammation (cytokines), might cause long-term inhibition of p53 activity.
Products of NF-κB-responsive genes can also suppress p53 function by acting against its downstream targets. For example, NF-κB-induced antiapoptotic factors can neutralize the effects of proapoptotic products induced by p53 (Perkins 2007). Also, proinflammatory NF-κB-induced cytokines (such as interleukin [IL]-6 and migration inhibitory factor[MIF]) may suppress p53’s transcriptional activity (Yonish-Rouach et al. 1991; Hudson et al. 1999; O’Prey et al. 2010).
In summary, the antagonism between p53 and NF-κB is likely regulated on multiple levels and through engagement of multiple mechanisms. It is important to underscore that, once NF-κB becomes constitutively active, p53 function is suppressed to the extent that it is no longer able to act as a tumor suppressor.
p53 AND SENESCENCE-RELATED CHRONIC INFLAMMATION: ROLE IN AGING AND CANCER
A gradual increase in systemic inflammation is one of the universal hallmarks of aging (inflammaging) (Franceschi et al. 2000a; Franceschi and Campisi 2014). Signs of this include elevation of serum cytokine levels, infiltration of immunocytes into tissues, and a high frequency of inflammation-associated diseases in elderly subjects (Schiffrin et al. 2010), which are all indicative of elevated NF-κB activity. Consistent with this and the reciprocal negative regulation of NF-κB and p53, the activity of p53 in mouse tissues gradually declines with age (Donehower 2009). As mentioned above, “inflammaging” may result from multiple causes, such as accumulation of products from damaged cells and organelles (mitochondria) or products of oral and gut microbiota, age-related changes to the immune and coagulation systems, and defective autophagy responses (Medzhitov 2008; Lopez-Otin et al. 2013; Franceschi and Campisi 2014). These alterations result in enhanced activation of the NLRP3 inflammasome and other proinflammatory pathways, ultimately leading to increased production of IL-1β, TNF, and IFNs (Lopez-Otin et al. 2013; Franceschi and Campisi 2014).
Cellular senescence is another important contributor to age-related inflammation (Fig. 1). Senescence is an epigenetic reprogramming of cells occurring in response to damage and stress and is characterized by irreversible growth arrest. A potent inducer of senescence is the response to DNA damage, and activation of the p53/21 pathway. Similar molecular events accompany establishment of senescence in normal cells transduced with oncogenic Ras—another senescence-inducing type of stress (Serrano et al. 1997).
Although p53-mediated growth arrest is an essential trigger for conversion of cells into a senescent state, additional events are required to make this arrest irreversible, such as the activation of the p16INK4a/pRB pathway (Beausejour et al. 2003). Involvement of mechanistic target of rapamycin (mTOR) pathway activity in the decision between reversible growth arrest and senescence is supported by the finding that the mTOR inhibitor rapamycin suppressed establishment of senescence (Demidenko et al. 2010; Korotchkina et al. 2010; Leontieva and Blagosklonny 2010). The inhibitory effect of p53 on mTOR is the most likely explanation for the antisenescence activity of p53 observed in several models (Demidenko et al. 2010; Korotchkina et al. 2010; Leontieva and Blagosklonny 2010). It is widely accepted that entry into a senescent state is one of the p53/Rb-dependent tumor-suppressor mechanisms that prevents malignant transformation and cancer by preventing proliferation of cells with a compromised genome or malfunctioning proto-oncogenes (Dimauro and David 2010).
Senescence is also characterized by acquisition of a so-called senescence-associated secretory phenotype (SASP)—constant secretion of a specific set of proinflammatory cytokines driven by constitutively active NF-κB (Campisi and Robert 2014). A normal physiological function of SASP has recently been suggested based on observations of a wound-healing role of senescent cells (Demaria et al. 2014). In addition, senescent cells sharing features of oncogene-induced senescence and SASP are present in embryos (Munoz-Espin et al. 2013; Storer et al. 2013). These discoveries suggest that cellular senescence is a form of terminal differentiation contributing to normal development and tissue regeneration.
Persistent senescent cells are also thought to drive aging and age-associated pathologies because of their SASP involving secretion of numerous proinflammatory cytokines that modify the tissue microenvironment and alter the function of nearby cells (Campisi and Robert 2014). NF-κB-driven transcription of cytokines and chemokines is a major component of this phenotype, determining, at least in part, the phenomenon of age-associated chronic inflammation (Chien et al. 2011). Senescent cells accumulate with age in many tissues. Using indicators of DNA-damage response and senescence-associated β-galactosidase expression as markers of senescent cells, an increase in the proportion of senescent cells with age was found in liver, skin, lung, and spleen of mice (Wang et al. 2009). No accumulation of senescent cells was found in heart, skeletal muscle, and kidney, which suggests that cellular senescence is not a general property of all tissues in aged organisms.
The potential importance of senescent cells in physiological aging is supported by a recent study showing absence of cellular senescence in naked mole rats, an exceptionally long-living rodent (Gorbunova et al. 2014). p53 deficiency greatly reduced the onset of premature aging in two genetic models of premature aging in mice (Cao et al. 2003; Varela et al. 2005). An even stronger argument was presented by Baker et al. (2011), who showed signs of reversion of several traits of progeroid phenotypes of mutant mice following pharmacological eradication of accumulated senescent cells expressing p16INK4a.
Thus, the same mechanism by which p53 promotes a long cancer-free life and effective wound healing (induction of cellular senescence) can actually become the source of procarcinogenic chronic systemic inflammation in aged organisms through excessive accumulation of senescent cells. This defines two roles for p53 in longevity, the relative impact of which gradually changes during the life of a mammal: (1) p53 reduces the risk of death from cancer in young organisms; but (2) with time, side effects of p53’s senescence-inducing activity are translated into chronic systemic inflammation that accelerates aging and provokes carcinogenicity.
This dual role of p53 is reflected by seemingly controversial observations of the phenotypes of mice with deregulated expression of p53. For example, the increased p53 gene dose in transgenic “super p53” mice carrying an extra copy of the p53 gene significantly protected them from cancer and did not result in premature aging (Garcia-Cao et al. 2002). Similarly, “super Ink4a/Arf” mice carrying an extra transgenic copy of the entire Ink4a/Arf locus have elevated p53 activity and show higher resistance to cancer than wild-type mice and normal aging and life span as well (Matheu et al. 2004). Consistent with this, mice in which Mdm2 expression was genetically reduced had a normal life span and were resistant to tumor development (Mendrysa et al. 2003, 2006). However, mice, in which overexpression of p53 was accompanied by an imbalance in the normal ratios of different p53 isoforms, showed an alarming premature aging phenotype (Maier et al. 2004). Also, several knockout and transgenic mouse lines that showed increased p53 activity had premature aging phenotypes (Matheu et al. 2008; Hinkal et al. 2009). In some cases, these aging phenotypes were partially rescued by reduction of the p53 dosage (Purdie et al. 1994; Donehower 2002).
These facts fit the following model. Senescent cells accumulate in tissues as a result of a two-step process. The first step (quiescence) is driven by p53-mediated growth arrest in response to aberrant proto-oncogene pathway activation and oxidative stress causing DNA damage. The second step makes growth arrest irreversible. It involves p16/Rb activation, depends on metabolic stimulation of quiescent cells, and requires mTOR/AKT activity; it can be effectively suppressed by the mTOR inhibitor rapamycin and by nongenotoxic activation of p53 (with Nutlin-3A) (Demidenko et al. 2010; Korotchkina et al. 2010; Leontieva and Blagosklonny 2010). The role of p53 is twofold: on one hand, it recruits cells into a quiescent state and, on the other hand, it inhibits their transition into senescence by suppressing mTOR/AKT pathway activity. With age, the proportion of senescent cells in tissues gradually increases, leading to elevated tissue concentrations of their secreted products (SASP) and establishment of inflammation. Inflammation, in turn, inhibits p53 activities, including its suppression of NF-κB and mTOR, and thereby elevates risk of cancer.
The proposed model accommodates Judy Campisi’s hypothesis linking aging with the SASP of accumulating senescent cells (Coppe et al. 2008), indications of a general decline in p53 function in aging tissues (Feng et al. 2007), the phenomenon of p53 suppression by NF-κB, and two distinct roles of p53: (1) its role as a suppressor of senescence via inhibition of mTOR/AKT (Feng et al. 2008; Feng and Levine 2010); and (2) its role as an inducer of growth arrest in response to oxidative or oncogenic stresses, which can eventually lead to irreversible senescence (Levine et al. 2006; Evan and d’Adda di Fagagna 2009). Moreover, this model provides a mechanistic basis for the observed age-related increase in cancer incidence, linking it to inflammation-mediated p53 suppression. It is consistent with the anti-aging effects of the mTOR inhibitor rapamycin, anti-inflammatory agents, and antioxidants, but clearly shows that these agents may be useful only for prophylaxis, not reversion, of aging-related syndromes. This model also predicts that restoration of p53 function by targeting the mechanism of NF-κB-mediated p53 suppression has the potential to slow down self-accelerating age-associated inflammation.
Accumulating evidence indicates that cellular senescence and associated inflammation can have a carcinogenic effect even outside of the context of aging. Senescent cells cocultured with tumor cells greatly facilitate tumor cell growth (Coppe et al. 2010a). Also, xenotransplantation of senescent cells with fully malignant cancer cells significantly accelerates the rate of tumor formation in mice (Liu and Hornsby 2007; Bhatia et al. 2008; Bartholomew et al. 2009; Coppe et al. 2010b). Recent work by Pribluda et al. (2013) showed that low-grade inflammation in stressed epithelium promoted tumorigenesis driven by cells with impaired p53 function. Treatment of mice with anti-inflammatory agents suppressed the senescence-induced inflammation and prevented carcinogenesis, constituting, as suggested by the investigators, a key mechanism in the anticarcinogenic effects of nonsteroidal anti-inflammatory drugs.
TARGETING THE MUTUAL ANTAGONISM OF p53 AND NF-κB TO TREAT DISEASES
Abnormal acute activity of p53 or NF-κB pathways can lead to lethal pathologies. Excessive induction of NF-κB, which can result from exposure to powerful microbial agonists of innate immunity receptors or injection of large doses of proinflammatory cytokines, can produce severe systemic acute inflammation known as septic shock, one of the most frequent causes of human death (Gustot 2011). Activation of p53 following systemic genotoxic stress caused by ionizing radiation or treatment with cytotoxic chemotherapeutic drugs results in massive p53-dependent apoptosis in sensitive tissues (Gudkov and Komarova 2010), which can result in development of acute radiation syndrome or severe side effects of cancer treatment (Gudkov and Komarova 2005). Chronic deregulation of either the p53 or NF-κB pathways can also be pathogenic. As previously mentioned, constitutive activation of NF-κB by extrinsic or intrinsic stimuli is the cause of diseases associated with chronic inflammation (Karin et al. 2002; Karin 2006). On the other hand, deficiencies in different branches of NF-κB signaling results in immunodeficiency. Even partial genetic deficiency in p53 function produces cancer-prone conditions in patients with Li–Fraumeni syndrome (Palmero et al. 2010).
Therefore, p53 and NF-κB inhibitors could have a substantial clinical impact for prevention and/or treatment of a number of serious pathologies (Dey et al. 2008; Coupienne et al. 2011; Suzuki et al. 2011). Moreover, the reciprocal negative regulation of p53 and NF-κB presents a potential opportunity for modulating the activity of each of these pathways by pharmacological targeting of its counterpart.
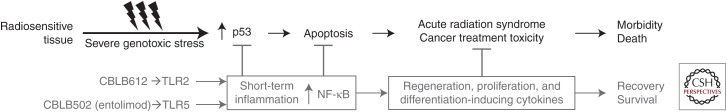
This is supported by our demonstration that acute radiation syndrome largely driven by p53-mediated apoptosis (Gudkov and Komarova 2010) and preventable by pharmacological inhibition of p53 (Komarov et al. 1999; Strom et al. 2006) can be effectively prevented and mitigated by NF-κB activators (Fig. 5). Specifically, we found that agonists of TLR2 (lipopeptides of mycoplasma) and, even more so, TLR5 (flagellin proteins of Gram-negative bacteria) act as powerful radioprotectants in mice (Burdelya et al. 2008; Shakhov et al. 2012). We developed a pharmacologically optimized derivative of Salmonella flagellin (named entolimod or CBLB502) and showed that it both protects against and mitigates radiation damage to the hematopoietic (HP) and gastrointestinal (GI) systems of lethally irradiated mice and Rhesus macaques (Burdelya et al. 2008). Dramatically improved survival of entolimod-treated irradiated animals was associated with strong suppression of p53-dependent apoptosis of HP precursors in the bone marrow and GI precursors in crypts of the small intestine (Burdelya et al. 2008), thus showing that the TLR5 agonist indeed acted as a suppressor of p53 function. Entolimod is currently in clinical development as a radiation antidote for use during nuclear emergencies and as a combined supportive care and immunotherapeutic drug for cancer patients (clinicaltrials.gov/ct2/show/NCT01527136). Importantly, radioprotection by both NF-κB-inducing TLR agonists such as entolimod and p53 inhibitors is not associated with any detectable increase in cancer frequency, indicating that short-term reversible inhibition of p53 function is safe (Komarov et al. 1999; Leonova et al. 2010).
Figure 5.
Short-term activation of NF-κB can neutralize tissue damage mediated by p53-dependent apoptosis following systemic genotoxic stress (ionizing radiation, chemotherapy with DNA-damaging agents). The scheme is applicable to radiosensitive tissues (e.g., hematopoietic system or epithelium of gastrointestinal [GI] tract). CBLB612 is a synthetic lipopeptide, TLR2 agonist, that mimics the activity of mycoplasma lipopeptide (Shakhov et al. 2010). CBLB502 (entolimod) is a recombinant protein, derivative of Salmonella flagellin, agonist of TLR5 (Burdelya et al. 2008).
On the other hand, stimulation of the NF-κB-suppressive activity of p53 can be considered as a strategy for treatment of diseases associated with excessive inflammation. The feasibility of this approach is supported by the properties of Nutlin-3A, a small molecule inhibitor of p53–Mdm2 interaction that is capable of nongenotoxic activation of p53 (Vassilev et al. 2004). Pharmacological agents acting through this mechanism are currently in clinical trials in cancer patients (Shangary and Wang 2008). Anti-inflammatory effects of Nutlin-3A were shown in vitro and in vivo (Dey et al. 2007; Groskreutz et al. 2007; Liu et al. 2009).
The mechanism of NF-κB-dependent p53 suppression frequently acquired by tumors (Gurova et al. 2005) opens the opportunity for development of anticancer drugs capable of simultaneous suppression of NF-κB and activation of p53. Such multitargeted drugs would be expected to have strong efficacy, broad applicability, and a low risk of drug-resistance development. The first drug candidate with this combination of properties, named curaxin CBL0137 (Gasparian et al. 2011), is currently in phase I clinical trials (clinicaltrials.gov/ct2/show/NCT0190522).
CONCLUDING REMARKS
Chronic inflammation is a major, if not “the” major, challenge that can potentially prevent p53 from exerting its tumor-suppressor function. Through long-term disabling of p53, the prolonged NF-κB activation associated with chronic inflammation can dramatically increase the risk of cancer development. Why would evolution tolerate or even support suppression of an organism’s major tumor-suppressor mechanism (p53 signaling) by its inflammatory response mechanism (NF-κB signaling)? As discussed in this review, accumulated evidence suggests that the answer to this question lies in the difficulty of coordinating organismal reactions to two types of stresses that require largely opposite types of responses (Fig. 4). p53 is the major cellular responder for intrinsic stress signals (e.g., DNA damage, oncogene expression, spindle poisoning, telomere shortening) for which a proapoptotic, antiproliferative outcome is the most beneficial to the organism (Cooks et al. 2014). Propagation of intrinsically damaged cells is restricted by internal cell decisions driven by p53. On the other hand, NF-κB is the major cellular responder for extrinsic stress signals (e.g., presence of infectious agents or traces—direct or indirect—of their presence). An altruistic self-restrictive and self-punishing response that may be appropriate for intrinsic disasters is not a rational strategy for responding to an extrinsic attack, which requires mobilization of all internal and external protective mechanisms. Hence, the benefit of suppression of p53 function by activated NF-κB for the organism likely lies in giving priority to the prosurvival pathway under conditions of the most severe stress. In fact, NF-κB responses deal with situations of extreme emergency (extrinsic assault by a variety of infectious agents), whereas p53 responses are aimed at preventing the more delayed, albeit at least equally serious, problem of cancer development. Hence, one would expect that under conditions requiring an NF-κB response (e.g., acute infectious disease), it would beneficial to impair p53 function to reduce unnecessary cell losses that might otherwise occur in tissues prone to p53-dependent apoptosis because of toxicities associated with infection. Unfortunately, what appears to have been originally designed by nature for rational and effective prioritization of responses to different types of stresses can produce disastrous outcomes when the mechanism of NF-κB-driven p53 suppression is misused and deregulated—as happens under conditions of chronic inflammation. However, by continuing to improve our understanding of this apparent flaw in the organization of mammalian organisms’ resistance to cancer, we will be able to devise new strategies to rationally disrupt the connection between chronic inflammation and cancer development and potentially reduce the incidence of cancer.
ACKNOWLEDGMENTS
We thank Patricia Stanhope Baker for valuable help in manuscript preparation. The authors’ work on p53 and inflammation was supported by Grants CA75179, AI080446, AI087616, and GM095874 from the National Institutes of Health (NIH), Cleveland BioLabs, and Everon Biosciences to A.V.G.
Footnotes
Editors: Guillermina Lozano and Arnold J. Levine
Additional Perspectives on The p53 Protein available at www.perspectivesinmedicine.org
REFERENCES
- Ak P, Levine AJ. 2010. p53 and NF-κB: Different strategies for responding to stress lead to a functional antagonism. FASEB J 24: 3643–3652. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase, which phosphorylates and activates protein kinase Bα. Curr Biol 7: 261–269. [DOI] [PubMed] [Google Scholar]
- Andoniou CE, Degli-Esposti MA. 2006. Insights into the mechanisms of CMV-mediated interference with cellular apoptosis. Immunol Cell Biol 84: 99–106. [DOI] [PubMed] [Google Scholar]
- Asquith M, Powrie F. 2010. An innately dangerous balancing act: Intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med 207: 1573–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. 1997. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89: 1175–1184. [DOI] [PubMed] [Google Scholar]
- Bai D, Ueno L, Vogt PK. 2009. Akt-mediated regulation of NF-κB and the essentialness of NF-κB for the oncogenicity of PI3K and Akt. Int J Cancer 125: 2863–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Coussens LM. 2004. Cancer: An inflammatory link. Nature 431: 405–406. [DOI] [PubMed] [Google Scholar]
- Bartholomew JN, Volonte D, Galbiati F. 2009. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res 69: 2878–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barykova YA, Logunov DY, Shmarov MM, Vinarov AZ, Fiev DN, Vinarova NA, Rakovskaya IV, Baker PS, Shyshynova I, Stephenson AJ, et al. 2011. Association of Mycoplasma hominis infection with prostate cancer. Oncotarget 2: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. 2003. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J 22: 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. 2011. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 12: 715–723. [DOI] [PubMed] [Google Scholar]
- Bhatia B, Multani AS, Patrawala L, Chen X, Calhoun-Davis T, Zhou J, Schroeder L, Schneider-Broussard R, Shen J, Pathak S, et al. 2008. Evidence that senescent human prostate epithelial cells enhance tumorigenicity: Cell fusion as a potential mechanism and inhibition by p16INK4a and hTERT. Int J Cancer 122: 1483–1495. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. 2007. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol 81: 1–5. [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. 2008. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell C, Zambetti GP. 2001. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene 277: 15–30. [DOI] [PubMed] [Google Scholar]
- Campbell KJ, Rocha S, Perkins ND. 2004. Active repression of antiapoptotic gene expression by RelA(p65) NF-κB. Mol Cell 13: 853–865. [DOI] [PubMed] [Google Scholar]
- Campisi J, Robert L. 2014. Cell senescence: Role in aging and age-related diseases. Interdiscip Top Gerontol 39: 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Li W, Kim S, Brodie SG, Deng CX. 2003. Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev 17: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MH. 2007. Hepatitis B virus infection. Semin Fetal Neonatal Med 12: 160–167. [DOI] [PubMed] [Google Scholar]
- Cheon H, Borden EC, Stark GR. 2014. Interferons and their stimulated genes in the tumor microenvironment. Semin Oncol 41: 156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao PJ, Miyamoto S, Verma IM. 1994. Autoregulation of IκBα activity. Proc Natl Acad Sci 91: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, et al. 2011. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 25: 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophorou MA, Martin-Zanca D, Soucek L, Lawlor ER, Brown-Swigart L, Verschuren EW, Evan GI. 2005. Temporal dissection of p53 function in vitro and in vivo. Nat Genet 37: 718–726. [DOI] [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. 2006. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443: 214–217. [DOI] [PubMed] [Google Scholar]
- Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, et al. 2013. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23: 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooks T, Harris CC, Oren M. 2014. Caught in the cross fire: p53 in inflammation. Carcinogenesis 35: 1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. 2010a. The senescence-associated secretory phenotype: The dark side of tumor suppression. Ann Rev Pathol 5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin K, Desprez PY, Campisi J. 2010b. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE 5: e9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupienne I, Bontems S, Dewaele M, Rubio N, Habraken Y, Fulda S, Agostinis P, Piette J. 2011. NF-κB inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochem Pharmacol 81: 606–616. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. 2002. Inflammation and cancer. Nature 420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241. [DOI] [PubMed] [Google Scholar]
- Davidson S, Maini MK, Wack A. 2015. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res 35: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DW, Weidner DA, Holian A, McConkey DJ. 2000. Nitric oxide-dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J Exp Med 192: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, et al. 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. 2010. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci 107: 9660–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. 2006. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6: 24–37. [DOI] [PubMed] [Google Scholar]
- Dey A, Wong ET, Bist P, Tergaonkar V, Lane DP. 2007. Nutlin-3 inhibits the NF-κB pathway in a p53-dependent manner: Implications in lung cancer therapy. Cell Cycle 6: 2178–2185. [DOI] [PubMed] [Google Scholar]
- Dey A, Tergaonkar V, Lane DP. 2008. Double-edged swords as cancer therapeutics: Simultaneously targeting p53 and NF-κB pathways. Nat Rev Drug Discov 7: 1031–1040. [DOI] [PubMed] [Google Scholar]
- Dimauro T, David G. 2010. Ras-induced senescence and its physiological relevance in cancer. Curr Cancer Drug Targets 10: 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA. 2002. Does p53 affect organismal aging? J Cell Physiol 192: 23–33. [DOI] [PubMed] [Google Scholar]
- Donehower LA. 2009. Using mice to examine p53 functions in cancer, aging, and longevity. Cold Spring Harb Perspect Biol 1: a001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. 2006. Lymphoid organ development: From ontogeny to neogenesis. Nat Immunol 7: 344–353. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. 2004. IκB-kinase β-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci 101: 2452–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon R, Rashi-Elkeles S, Lerenthal Y, Linhart C, Tenne T, Amariglio N, Rechavi G, Shamir R, Shiloh Y. 2005. Dissection of a DNA-damage-induced transcriptional network using a combination of microarrays, RNA interference and computational promoter analysis. Genome Biol 6: R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, d’Adda di Fagagna F. 2009. Cellular senescence: Hot or what? Curr Opin Genet Dev 19: 25–31. [DOI] [PubMed] [Google Scholar]
- Eymin B, Claverie P, Salon C, Leduc C, Col E, Brambilla E, Khochbin S, Gazzeri S. 2006. p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol Cell Biol 26: 4339–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Levine AJ. 2010. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol 20: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ. 2007. Declining p53 function in the aging process: A possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci 104: 16633–16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Hu W, Rajagopal G, Levine AJ. 2008. The tumor suppressor p53: Cancer and aging. Cell Cycle 7: 842–847. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69: S4–S9. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. 2000a. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 908: 244–254. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis G. 2000b. The network and the remodeling theories of aging: Historical background and new perspectives. Exp Gerontol 35: 879–896. [DOI] [PubMed] [Google Scholar]
- Frank AK, Leu JI, Zhou Y, Devarajan K, Nedelko T, Klein-Szanto A, Hollstein M, Murphy ME. 2011. The codon 72 polymorphism of p53 regulates interaction with NF-κB and transactivation of genes involved in immunity and inflammation. Mol Cell Biol 31: 1201–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275: 665–668. [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. 2002. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 21: 6225–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparian AV, Burkhart CA, Purmal AA, Brodsky L, Pal M, Saranadasa M, Bosykh DA, Commane M, Guryanova OA, Pal S, et al. 2011. Curaxins: Anticancer compounds that simultaneously suppress NF-κB and activate p53 by targeting FACT. Sci Transl Med 3: 95ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand M, Weinberg RW, Castle WM. 1967. Relation between carcinoma of the bladder and infestation with Schistosoma haematobium. Lancet 1: 1249–1251. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. 1997. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci 94: 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Mendoza T, Ortiz LA, Hoyle GW, Fermin CD, Brody AR, Friedman M, Morris GF. 2002. Bleomycin sensitivity of mice expressing dominant-negative p53 in the lung epithelium. Am J Respir Crit Care Med 166: 890–897. [DOI] [PubMed] [Google Scholar]
- Goh AM, Coffill CR, Lane DP. 2006. The role of mutant p53 in human cancer. J Pathol 223: 116–126. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Marcel V, Olivier M, Oren M, Rotter V, Hainaut P. 2011. Understanding wild-type and mutant p53 activities in human cancer: New landmarks on the way to targeted therapies. Cancer Gene Ther 18: 2–11. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Navajas JM, Lee J, David M, Raz E. 2012. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. 2014. Comparative genetics of longevity and cancer: Insights from long-lived rodents. Nat Rev Genet 15: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M. 2008. Inflammaging (inflammation+aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Bioscience Trends 2: 218–230. [PubMed] [Google Scholar]
- Graham DY. 2015. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 148: 719–731.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. 2010. Immunity, inflammation, and cancer. Cell 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groskreutz DJ, Monick MM, Yarovinsky TO, Powers LS, Quelle DE, Varga SM, Look DC, Hunninghake GW. 2007. Respiratory syncytial virus decreases p53 protein to prolong survival of airway epithelial cells. J Immunol 179: 2741–2747. [DOI] [PubMed] [Google Scholar]
- Gu L, Findley HW, Zhou M. 2002. MDM2 induces NF-κB/p65 expression transcriptionally through Sp1-binding sites: A novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood 99: 3367–3375. [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA. 2005. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun 331: 726–736. [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA. 2010. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol 2: a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov AV, Gurova KV, Komarova EA. 2011. Inflammation and p53: A tale of two stresses. Genes Cancer 2: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara NV, Kim HS, Antonova EI, Chan L. 1999. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat Med 5: 335–339. [DOI] [PubMed] [Google Scholar]
- Gulati AP, Yang YM, Harter D, Mukhopadhyay A, Aggarwal BB, Benzil DL, Whysner J, Albino AP, Murali R, Jhanwar-Uniyal M. 2006. Mutant human tumor suppressor p53 modulates the activation of mitogen-activated protein kinase and nuclear factor-κB, but not c-Jun N-terminal kinase and activated protein-1. Mol Carcinog 45: 26–37. [DOI] [PubMed] [Google Scholar]
- Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, Tararova ND, et al. 2005. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-κB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci 102: 17448–17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustot T. 2011. Multiple organ failure in sepsis: Prognosis and role of systemic inflammatory response. Curr Opin Crit Care 17: 153–159. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr. 1999. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19: 5785–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkal GW, Gatza CE, Parikh N, Donehower LA. 2009. Altered senescence, apoptosis, and DNA damage response in a mutant p53 model of accelerated aging. Mech Ageing Dev 130: 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel B, Schmid JA. 2013. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Yasuda H. 1999. Association of p19ARF with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J 18: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Ju TK, Hung MC, Chen CC. 2007. Phosphorylation of CBP by IKK-α promotes cell growth by switching the binding preference of CBP from p53 to NF-κB. Mol Cell 26: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. 1999. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 190: 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, et al. 2000. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res 60: 3333–3337. [PubMed] [Google Scholar]
- Ikeda A, Sun X, Li Y, Zhang Y, Eckner R, Doi TS, Takahashi T, Obata Y, Yoshioka K, Yamamoto K. 2000. p300/CBP-dependent and -independent transcriptional interference between NF-κB RelA and p53. Biochem Biophys Res Commun 272: 375–379. [DOI] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. 2013. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. 1997. An increased prevalence of Epstein–Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest 100: 3019–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke RU, Sohn D, Schulze-Osthoff K. 2008. The dark side of a tumor suppressor: Anti-apoptotic p53. Cell Death Differ 15: 959–976. [DOI] [PubMed] [Google Scholar]
- Janssens S, Tschopp J. 2006. Signals from within: The DNA-damage-induced NF-κB response. Cell Death Differ 13: 773–784. [DOI] [PubMed] [Google Scholar]
- Karin M. 2006. Nuclear factor-κB in cancer development and progression. Nature 441: 431–436. [DOI] [PubMed] [Google Scholar]
- Karin M. 2009. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1: a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. 2002. NF-κB in cancer: From innocent bystander to major culprit. Nat Rev Cancer 2: 301–310. [DOI] [PubMed] [Google Scholar]
- Kashatus D, Cogswell P, Baldwin AS. 2006. Expression of the Bcl-3 proto-oncogene suppresses p53 activation. Genes Dev 20: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. 2008. p53 regulates glucose metabolism through an IKK-NF-κB pathway and inhibits cell transformation. Nat Cell Biol 10: 611–618. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. 2009. Loss of p53 enhances catalytic activity of IKK-β through O-linked β-N-acetyl glucosamine modification. Proc Natl Acad Sci 106: 3431–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosawa K, Akahane Y, Nagata A, Furuta S. 1984. Hepatocellular carcinoma after non-A, non-B posttransfusion hepatitis. Am J Gastroenterol 79: 777–781. [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. 1999. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285: 1733–1737. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, Gudkov AV. 2004. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 23: 3265–3271. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin O, Kuprash DV, et al. 2005. p53 is a suppressor of inflammatory response in mice. FASEB J 19: 1030–1032. [DOI] [PubMed] [Google Scholar]
- Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. 2010. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Adami HO, Trichopoulos D. 2000. Infections as a major preventable cause of human cancer. J Int Med 248: 171–183. [DOI] [PubMed] [Google Scholar]
- Kutok JL, Wang F. 2006. Spectrum of Epstein–Barr virus-associated diseases. Annu Rev Pathol 1: 375–404. [DOI] [PubMed] [Google Scholar]
- Lane D, Levine A. 2010. p53 Research: The past thirty years and the next thirty years. Cold Spring Harb Perspect Biol 2: a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. 2004. Gain of function of a p53 hot spot mutation in a mouse model of Li–Fraumeni syndrome. Cell 119: 861–872. [DOI] [PubMed] [Google Scholar]
- La Rosa FA, Pierce JW, Sonenshein GE. 1994. Differential regulation of the c-myc oncogene promoter by the NF-κB rel family of transcription factors. Mol Cell Biol 14: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassus P, Ferlin M, Piette J, Hibner U. 1996. Anti-apoptotic activity of low levels of wild-type p53. EMBO J 15: 4566–4573. [PMC free article] [PubMed] [Google Scholar]
- Lee TL, Yang XP, Yan B, Friedman J, Duggal P, Bagain L, Dong G, Yeh NT, Wang J, Zhou J, et al. 2007. A novel nuclear factor-κB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clin Cancer Res 13: 5680–5691. [DOI] [PubMed] [Google Scholar]
- Leonova KI, Shneyder J, Antoch MP, Toshkov IA, Novototskaya LR, Komarov PG, Komarova EA, Gudkov AV. 2010. A small molecule inhibitor of p53 stimulates amplification of hematopoietic stem cells but does not promote tumor development in mice. Cell Cycle 9: 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonova KI, Brodsky L, Lipchick B, Pal M, Novototskaya L, Chenchik AA, Sen GC, Komarova EA, Gudkov AV. 2013. p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs. Proc Natl Acad Sci 110: E89–E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Blagosklonny MV. 2010. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2: 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Oren M. 2009. The first 30 years of p53: Growing ever more complex. Nat Rev Cancer 9: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hu W, Feng Z. 2006. The P53 pathway: What questions remain to be explored? Cell Death Differ 13: 1027–1036. [DOI] [PubMed] [Google Scholar]
- Li L, Ng DS, Mah W, Almeida FF, Rahmat SA, Rao VK, Leow SC, Laudisi F, Peh MT, Goh AM, et al. 2014. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ 22: 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. 2007. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 117: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. 2007. Fibroblast stimulation of blood vessel development and cancer cell invasion in a subrenal capsule xenograft model: Stress-induced premature senescence does not increase effect. Neoplasia 9: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. 2009. p53 attenuates lipopolysaccharide-induced NF-κB activation and acute lung injury. J Immunol 182: 5063–5071. [DOI] [PubMed] [Google Scholar]
- Loffeld RJ, Willems I, Flendrig JA, Arends JW. 1990. Helicobacter pylori and gastric carcinoma. Histopathology 17: 537–541. [DOI] [PubMed] [Google Scholar]
- Logunov DY, Scheblyakov DV, Zubkova OV, Shmarov MM, Rakovskaya IV, Gurova KV, Tararova ND, Burdelya LG, Naroditsky BS, Ginzburg AL, et al. 2008. Mycoplasma infection suppresses p53, activates NF-κB and cooperates with oncogenic Ras in rodent fibroblast transformation. Oncogene 27: 4521–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153: 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Kamata H, Karin M. 2005. The anti-death machinery in IKK/NF-κB signaling. J Clin Immunol 25: 541–550. [DOI] [PubMed] [Google Scholar]
- Madenspacher JH, Azzam KM, Gowdy KM, Malcolm KC, Nick JA, Dixon D, Aloor JJ, Draper DW, Guardiola JJ, Shatz M, et al. 2013. p53 Integrates host defense and cell fate during bacterial pneumonia. J Exp Med 210: 891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. 2004. Modulation of mammalian life span by the short isoform of p53. Genes Dev 18: 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cruz AB, Santos M, Garcia-Escudero R, Moral M, Segrelles C, Lorz C, Saiz C, Buitrago-Perez A, Costa C, Paramio JM. 2009. Spontaneous tumor formation in Trp53-deficient epidermis mediated by chromosomal instability and inflammation. Anticancer Res 29: 3035–3042. [PubMed] [Google Scholar]
- Matheu A, Pantoja C, Efeyan A, Criado LM, Martin-Caballero J, Flores JM, Klatt P, Serrano M. 2004. Increased gene dosage of Ink4a/Arf results in cancer resistance and normal aging. Genes Dev 18: 2736–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Serrano M. 2008. The Arf/p53 pathway in cancer and aging. Cancer Res 68: 6031–6034. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature 454: 428–435. [DOI] [PubMed] [Google Scholar]
- Mendrysa SM, McElwee MK, Michalowski J, O’Leary KA, Young KM, Perry ME. 2003. mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol 23: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrysa SM, O’Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME. 2006. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev 20: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Williams E, Chan L. 2003. Macrophage-specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler Thromb Vasc Biol 23: 1608–1614. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Lounsbury KM, Reddy SP. 2006. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am J Respir Cell Mol Biol 34: 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Vousden KH. 2013. p53 mutations in cancer. Nat Cell Biol 15: 2–8. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. 2013. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Komarov AP, Ippolito T, Bonneau K, Chenchik AA, Gudkov AV. 2014. Peptides genetically selected for NF-κB activation cooperate with oncogene Ras and model carcinogenic role of inflammation. Proc Natl Acad Sci 111: E474–E483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. 2006. Mechanisms of disease: Proatherogenic HDL—An evolving field. Nat Clin Pract Endocrinol Metab 2: 504–511. [DOI] [PubMed] [Google Scholar]
- Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. 2002. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem 277: 21843–21850. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Okuda M, Bernard CC. 2003. Regulatory role of p53 in experimental autoimmune encephalomyelitis. J Neuroimmunol 135: 29–37. [DOI] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. 2004. Mutant p53 gain of function in two mouse models of Li–Fraumeni syndrome. Cell 119: 847–860. [DOI] [PubMed] [Google Scholar]
- O’Prey J, Crighton D, Martin AG, Vousden KH, Fearnhead HO, Ryan KM. 2010. p53-mediated induction of Noxa and p53AIP1 requires NF-κB. Cell Cycle 9: 947–952. [DOI] [PubMed] [Google Scholar]
- Oren M, Rotter V. 2010. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol 2: a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmero EI, Achatz MI, Ashton-Prolla P, Olivier M, Hainaut P. 2010. Tumor protein 53 mutations and inherited cancer: Beyond Li–Fraumeni syndrome. Curr Opin Oncol 22: 64–69. [DOI] [PubMed] [Google Scholar]
- Park JS, Lim MA, Cho ML, Ryu JG, Moon YM, Jhun JY, Byun JK, Kim EK, Hwang SY, Ju JH, et al. 2013. p53 controls autoimmune arthritis via STAT-mediated regulation of the Th17 cell/Treg cell balance in mice. Arthritis Rheum 65: 949–959. [DOI] [PubMed] [Google Scholar]
- Perkins ND. 1997. Achieving transcriptional specificity with NF-κB. Int J Biochem Cell Biol 29: 1433–1448. [DOI] [PubMed] [Google Scholar]
- Perkins ND. 2007. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol 8: 49–62. [DOI] [PubMed] [Google Scholar]
- Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, Burstain I, Morgenstern Y, Brachya G, Billauer H, et al. 2013. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 24: 242–256. [DOI] [PubMed] [Google Scholar]
- Purdie CA, Harrison DJ, Peter A, Dobbie L, White S, Howie SE, Salter DM, Bird CC, Wyllie AH, Hooper ML, et al. 1994. Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene 9: 603–609. [PubMed] [Google Scholar]
- Ravi R, Mookerjee B, van Hensbergen Y, Bedi GC, Giordano A, El-Deiry WS, Fuchs EJ, Bedi A. 1998. p53-mediated repression of nuclear factor-κB RelA via the transcriptional integrator p300. Cancer Res 58: 4531–4536. [PubMed] [Google Scholar]
- Rocha S, Garrett MD, Campbell KJ, Schumm K, Perkins ND. 2005. Regulation of NF-κB and p53 through activation of ATR and Chk1 by the ARF tumour suppressor. EMBO J 24: 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Kono H. 2008. The inflammatory response to cell death. Ann Rev Pathol 3: 99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Dalgleish A. 2011. Infection, immunoregulation, and cancer. Immunol Rev 240: 141–159. [DOI] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. 2000. Role of NF-κB in p53-mediated programmed cell death. Nature 404: 892–897. [DOI] [PubMed] [Google Scholar]
- Salvador JM, Hollander MC, Nguyen AT, Kopp JB, Barisoni L, Moore JK, Ashwell JD, Fornace AJ Jr. 2002. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity 16: 499–508. [DOI] [PubMed] [Google Scholar]
- Salvador JM, Mittelstadt PR, Belova GI, Fornace AJ Jr, Ashwell JD. 2005. The autoimmune suppressor Gadd45α inhibits the T cell alternative p38 activation pathway. Nat Immunol 6: 396–402. [DOI] [PubMed] [Google Scholar]
- Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS, Corbett JA, Egan TM, et al. 2009. The CDK domain of p21 is a suppressor of IL-1β-mediated inflammation in activated macrophages. Eur J Immunol 39: 820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. 2010. The inflammatory status of the elderly: The intestinal contribution. Mutat Res 690: 50–56. [DOI] [PubMed] [Google Scholar]
- Schneider G, Kramer OH. 2011. NF-κB/p53 crosstalk—A promising new therapeutic target. Biochim Biophys Acta 1815: 90–103. [DOI] [PubMed] [Google Scholar]
- Schneider G, Henrich A, Greiner G, Wolf V, Lovas A, Wieczorek M, Wagner T, Reichardt S, von Werder A, Schmid RM, et al. 2010. Cross talk between stimulated NF-κB and the tumor suppressor p53. Oncogene 29: 2795–2806. [DOI] [PubMed] [Google Scholar]
- Schumm K, Rocha S, Caamano J, Perkins ND. 2006. Regulation of p53 tumour suppressor target gene expression by the p52 NF-κB subunit. EMBO J 25: 4820–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA. 1980. Helminths in the induction of cancer: Opisthorchis viverrini, Clonorchis sinensis and cholangiocarcinoma. Trop Geogr Med 32: 95–100. [PubMed] [Google Scholar]
- Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. 2004. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 64: 2627–2633. [DOI] [PubMed] [Google Scholar]
- Scian MJ, Stagliano KE, Anderson MA, Hassan S, Bowman M, Miles MF, Deb SP, Deb S. 2005. Tumor-derived p53 mutants induce NF-κB2 gene expression. Mol Cell Biol 25: 10097–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602. [DOI] [PubMed] [Google Scholar]
- Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, et al. 2012. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS ONE 7: e33044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangary S, Wang S. 2008. Targeting the MDM2–p53 interaction for cancer therapy. Clin Cancer Res 14: 5318–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HM, Tergaonkar V. 2009. NF-κB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis 14: 348–363. [DOI] [PubMed] [Google Scholar]
- Sherlock S, Fox RA, Niazi SP, Scheuer PJ. 1970. Chronic liver disease and primary liver-cell cancer with hepatitis-associated (Australia) antigen in serum. Lancet 1: 1243–1247. [DOI] [PubMed] [Google Scholar]
- Sigal A, Rotter V. 2000. Oncogenic mutations of the p53 tumor suppressor: The demons of the guardian of the genome. Cancer Res 60: 6788–6793. [PubMed] [Google Scholar]
- Stolte M. 1992. Helicobacter pylori gastritis and gastric MALT-lymphoma. Lancet 339: 745–746. [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al. 2013. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130. [DOI] [PubMed] [Google Scholar]
- Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, et al. 2006. Small-molecule inhibitor of p53 binding to mitochondria protects mice from γ radiation. Nat Chem Biol 2: 474–479. [DOI] [PubMed] [Google Scholar]
- Strozyk E, Poppelmann B, Schwarz T, Kulms D. 2006. Differential effects of NF-κB on apoptosis induced by DNA-damaging agents: The type of DNA damage determines the final outcome. Oncogene 25: 6239–6251. [DOI] [PubMed] [Google Scholar]
- Sunami Y, Wirth T. 2011. Intestinal carcinogenesis: IKK can go all the way. J Clin Invest 121: 2551–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Ogawa M, Muto S, Itai A, Isobe M, Hirata Y, Nagai R. 2011. Novel IκB kinase inhibitors for treatment of nuclear factor-B-related diseases. Expert Opin Investig Drugs 20: 395–405. [DOI] [PubMed] [Google Scholar]
- Szoltysek K, Pietranek K, Kalinowska-Herok M, Pietrowska M, Kimmel M, Widlak P. 2008. TNF-α-induced activation of NF-κB protects against UV-induced apoptosis specifically in p53-proficient cells. Acta Biochim Pol 55: 741–748. [PubMed] [Google Scholar]
- Tavana O, Benjamin CL, Puebla-Osorio N, Sang M, Ullrich SE, Ananthaswamy HN, Zhu C. 2010. Absence of p53-dependent apoptosis leads to UV radiation hypersensitivity, enhanced immunosuppression and cellular senescence. Cell Cycle 9: 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergaonkar V, Perkins ND. 2007. p53 and NF-κB crosstalk: IKK-α tips the balance. Mol Cell 26: 158–159. [DOI] [PubMed] [Google Scholar]
- Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. 2002. p53 stabilization is decreased upon NF-κB activation: A role for NF-κB in acquisition of resistance to chemotherapy. Cancer Cell 1: 493–503. [DOI] [PubMed] [Google Scholar]
- Thompson MP, Kurzrock R. 2004. Epstein–Barr virus and cancer. Clin Cancer Res 10: 803–821. [DOI] [PubMed] [Google Scholar]
- Trakala M, Arias CF, Garcia MI, Moreno-Ortiz MC, Tsilingiri K, Fernandez PJ, Mellado M, Diaz-Meco MT, Moscat J, Serrano M, et al. 2009. Regulation of macrophage activation and septic shock susceptibility via p21(WAF1/CIP1). Eur J Immunol 39: 810–819. [DOI] [PubMed] [Google Scholar]
- Ullman TA, Itzkowitz SH. 2011. Intestinal inflammation and cancer. Gastroenterology 140: 1807–1816. [DOI] [PubMed] [Google Scholar]
- Vaid M, Katiyar SK. 2010. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.). Int J Oncol 36: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, et al. 2001. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ Res 88: 780–786. [DOI] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, et al. 2005. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437: 564–568. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. 2004. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848. [DOI] [PubMed] [Google Scholar]
- Vaughan CA, Singh S, Windle B, Sankala HM, Graves PR, Andrew Yeudall W, Deb SP, Deb S. 2012. p53 mutants induce transcription of NF-κB2 in H1299 cells through CBP and STAT binding on the NF-κB2 promoter and gain of function activity. Arch Biochem Biophys 518: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Thusen JH, van Vlijmen BJ, Hoeben RC, Kockx MM, Havekes LM, van Berkel TJ, Biessen EA. 2002. Induction of atherosclerotic plaque rupture in apolipoprotein E–/– mice after adenovirus-mediated transfer of p53. Circulation 105: 2064–2070. [DOI] [PubMed] [Google Scholar]
- Wadgaonkar R, Phelps KM, Haque Z, Williams AJ, Silverman ES, Collins T. 1999. CREB-binding protein is a nuclear integrator of nuclear factor-κB and p53 signaling. J Biol Chem 274: 1879–1882. [DOI] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8: 311–323. [DOI] [PubMed] [Google Scholar]
- Webster GA, Perkins ND. 1999. Transcriptional cross talk between NF-κB and p53. Mol Cell Biol 19: 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz L, Damalas A, Liontos M, Karakaidos P, Fontemaggi G, Maor-Aloni R, Kalis M, Levrero M, Strano S, Gorgoulis VG, et al. 2007. Mutant p53 enhances nuclear factor κB activation by tumor necrosis factor α in cancer cells. Cancer Res 67: 2396–2401. [DOI] [PubMed] [Google Scholar]
- Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. 2009. Phosphorylation of p53 by IκB kinase 2 promotes its degradation by β-TrCP. Proc Natl Acad Sci 106: 2629–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, Zvaifler NJ, Green DR, Firestein GS. 2002. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol 160: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. 2004. Physiological functions of protein kinase B/Akt. Biochem Soc Trans 32: 350–354. [DOI] [PubMed] [Google Scholar]
- Yeudall WA, Vaughan CA, Miyazaki H, Ramamoorthy M, Choi MY, Chapman CG, Wang H, Black E, Bulysheva AA, Deb SP, et al. 2012. Gain-of-function mutant p53 upregulates CXC chemokines and enhances cell migration. Carcinogenesis 33: 442–451. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. 1991. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352: 345–347. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH. 2005. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes 54: 1423–1428. [DOI] [PubMed] [Google Scholar]