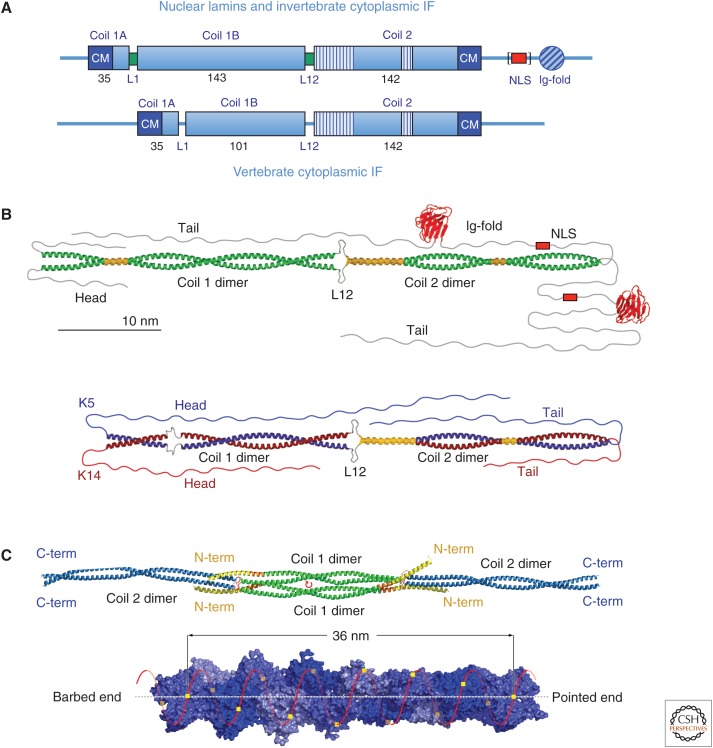
Figure 1.
Structural organization of intermediate filament (IF) proteins. (A) Domain organization of nuclear lamin and invertebrate cytoplasmic (top) and vertebrate cytoplasmic (bottom) IF proteins. Helical segments are designated “coil” and drawn as boxes, with the numbers represent the amino acid length of each segment. The striped parts of coil 2 indicate regions with hendecad repeats instead of the standard heptad repeats, which mediate coiled-coil formation. The red box designates the nuclear localization signal (NLS)—this is found in lamins, but not in invertebrate cytoplasmic IF proteins (hence, it is bracketed in the figure). The “Ig-fold” is a structurally conserved domain of approximately 100 amino acids showing an immunoglobulin-like fold found in nearly all nuclear lamins and most invertebrate cytoplasmic IF proteins. “CM” indicates conserved amino acid sequence motifs that occur at the amino-terminal and carboxy-terminal end of the rod, referred to as IF consensus motif 1 and 2, respectively. L1 and L12 indicate intrinsically disordered linker segments connecting the corresponding helices, although in lamins they can adopt an ordered state (green boxes). (B) Structural model of a lamin A homodimer (top) and a keratin heterodimer formed by K5 and K14 (bottom); the α-helical segments colored yellow represent parallel helices or “paired bundles”—the short paired bundle in the middle of the coil 2 domain has also been referred to as a “stutter.” (Redrawn from Herrmann et al. 2009, with permission from the American Society for Clinical Investigation.) (C) (Top) molecular structure of a vimentin tetramer featuring an antiparallel, half-staggered alignment of two coiled-coil dimers via coil 1 in the A11 mode. The center of symmetry is indicated by a small red circular arrow. (Adapted from Chernyatina et al. 2012). C-term, carboxyl terminal; N-term, amino terminal. (Bottom) A 14-subunit-long actin filament segment showing polar helical packing of 13 subunits in six left-handed turns with an axial increase of 2.75 nm per subunit. (Adapted from Dominguez and Holmes 2011, © Annual Reviews; http://www.annualreviews.org.)