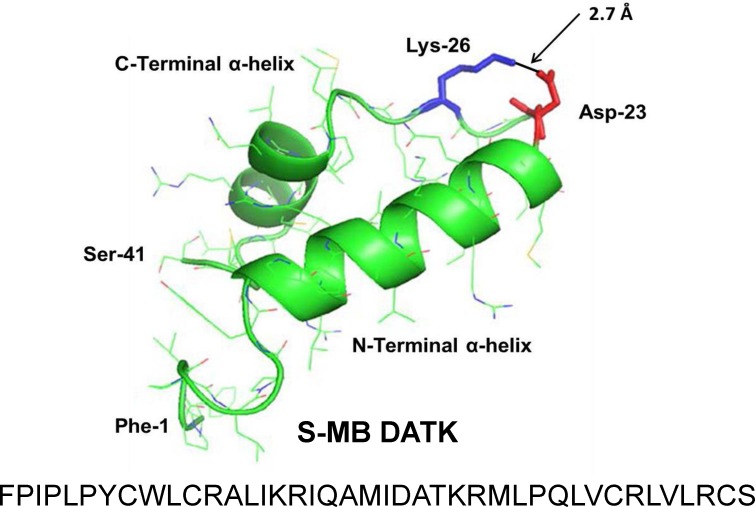
Figure 1. Linear sequence and homology-modeled ribbon structure of Super Mini-B (S-MB) DATK peptide.
The figure shows the homology modeled structure of the 41 residue sequence of S-MB DATK (Notter, Wang & Walther, 2016). This active mimic of human SP-B contains its functional amphipathic N- and C-terminal helices, lipophilic insertion sequence, and primary saposin fold character plus a structure-stabilizing DATK substitution. Helical residues in the homology modeled peptide structure are highlighted in green ribbon, with disordered and loop-turn segments represented as green tubes. The turn-stabilizing ion-pair is rendered in red (Asp−-23) and blue (Lys+-26), with a calculated equilibrium separation distance of 2.7 Å. The characteristic “saposin fold” of reduced S-MB DATK encompasses the N-terminal α-helix (residues 8–21), the loop-turn (residues 22–29) and C-terminal α-helix (residues 30–37), while the additional lipophilic N-terminal insertion sequence includes residues 1–7.