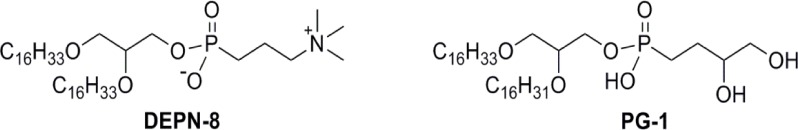
Figure 3. Schematic structures of phospholipase-resistant phosphonolipid analogs DEPN-8 and PG-1.
DEPN-8 is a disaturated diether phosphonolipid analog of DPPC (C16:0, C16:0), while PG-1 is a mono-unsaturated diether phosphonolipid analog of POPG (C16:0, C16:1). The presence of ether rather than ester linkages at the chain-backbone junctions of DEPN-8 and PG-1, together with their headgroup phosphonate rather than phosphate moiety, make these analog compounds inherently structurally-resistant to chemical degradation by phospholipases A1, A2, and D. DEPN-8 is also partially resistant to phospholipase C due to steric hindrance. See text for details.