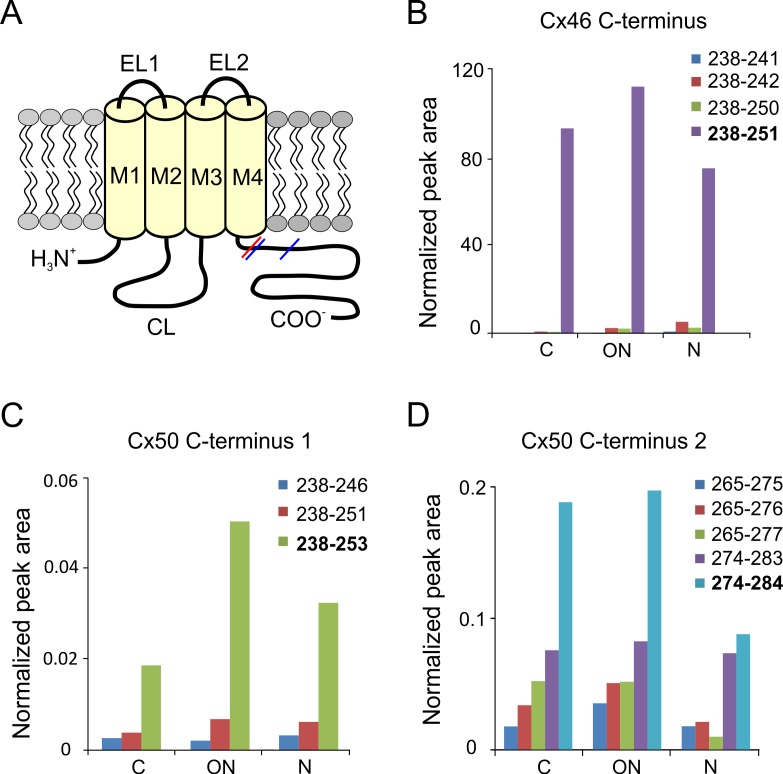
Figure 2.
Cleavage sites in the C-termini of Cx46 and Cx50 in a 55-year-old human lens. (A) Schematic drawing of the connexin membrane topology indicating the sites of truncation in Cx46 (red) and Cx50 (blue) in the C-terminus. CL, cytoplasmic loop; EL1, EL2, extracellular domains; TM1–TM4, transmembrane domains. Truncation of the C-termini was found in samples obtained from the cortex (C), outer nucleus (ON), and the nucleus (N). Cleavage sites adjacent to the fourth transmembrane domain were found in Cx46 (B) and Cx50 (C). Peak areas of nontryptic Cx46 peptides (B) were normalized to peak area of Cx46 peptide 10 to 23 because the full-length tryptic peptide was too long to show good signal in MS. Peak areas for Cx50 peptides (C) were normalized to peak area of Cx50 peptide 238 to 250. Truncation at H253 and S251 results in very short, undetectable tryptic peptides due to a lysine at position 250; therefore, only the miscleaved tryptic peptides (238–251 and 238–253) were detected as truncation products. Because the normalized peak areas shown in (C) were calculated by comparing the miscleaved truncated peptides to the fully tryptic peptide (251–262), differences in ionization efficiencies could occur and, therefore, the low normalized peak areas do not necessarily indicate a low prevalence of truncation at these sites. The Cx50 C-terminus was also cleaved at multiple locations between residues 274 and 284 (D), and the signal was normalized to peak area of Cx50 265 to 273. The major truncated products are indicated in bold. The relative abundance of Cx46 and Cx50 truncations did not change in the different regions of the lens.