Abstract
Practicing physicians have requested efficacy and safety data for belimumab, when used with specific systemic lupus erythematosus (SLE) medications. This was a post hoc analysis of pooled efficacy and safety data from patients who received belimumab 10 mg/kg plus standard of care (SoC) or placebo (SoC) in two Phase III, randomized trials, BLISS-52 and BLISS-76. Patients were categorized into four groups based on baseline concomitant medication usage: steroids only; antimalarials (AM) only; steroids + AM; or steroids + AM + immunosuppressants (IS). The primary endpoint was the SLE Responder Index (SRI) at Week 52. SRI over time and individual SRI components were secondary endpoints. Time to first flare and changes in concomitant medications were exploratory endpoints. Safety was assessed using adverse event (AE) reporting. Across 834 patients, steroids + AM was the largest group (n = 346, 41.5%) and AM only was the smallest (n = 77, 9.2%). Disease duration was shortest in the steroids + AM group (5.7 years vs 6.4–7.1 years); SELENA-SLEDAI scores were similar across groups. At Week 52, the percentage of SRI responders was greatest in the steroids + AM group for belimumab 10 mg/kg (59%) compared with placebo (44%); treatment response and SRI component improvements were also observed across other groups. The probability of experiencing an SLE flare was reduced in the steroids-only group for patients who received belimumab 10 mg/kg compared with placebo (64.3% vs 78.1%; hazard ratio 0.64; 95% confidence interval: 0.42–0.96). There was little or no change in daily AM or IS dose in any group. For all groups, there was a general decrease in steroid dose over time; a quarter to a third of patients experienced decreased steroid doses at Week 52. The overall safety profile was similar across treatment arms and concomitant medication groups, with the exception of serious AEs in the steroids + AM group (belimumab 10 mg/kg 16%, placebo 8%). The efficacy and safety of belimumab in combination with SoC was demonstrated for various groupings of steroids, AM and IS. These findings may improve the understanding of the safety and efficacy of adding belimumab to different treatments.
Keywords: Systemic lupus erythematosus, SLE Responder Index, belimumab, concomitant medications
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease associated with increased morbidity and mortality.1 The clinical manifestations of SLE are varied and affect several organ systems; renal disease and skin and musculoskeletal involvement are frequently reported.1 SLE has a complex immunological pathology, culminating in the deposition of autoantibodies and ensuing tissue damage.2 For many years, lupus standard of care (SoC) has routinely included antimalarials (AM), immunosuppressants (IS) and steroids, alone or in combination.1
In 2011, the recombinant human monoclonal antibody, belimumab, was licensed for intravenous (IV) use in autoantibody-positive adults with active SLE as add-on therapy to SoC.3 Belimumab targets and inhibits the cytokine B lymphocyte stimulator (BLyS),4 which is involved in the selection and survival of B cells and thus production of autoantibodies.5 The efficacy and safety of belimumab have been demonstrated in two large, multicenter, randomized, controlled trials, A Study of Belimumab in Subjects with Systemic Lupus Erythematosus (BLISS)-526 and BLISS-76.7 The patient population included adults with active SLE who were autoantibody positive, and receiving SoC. The primary efficacy endpoint was the SLE Responder Index (SRI)8 response rate at Week 52.
As belimumab is prescribed as an add-on therapy to SoC, physicians have requested data regarding the efficacy and safety of belimumab 10 mg/kg in combination with typical groupings of concomitant SLE medications. We conducted a post hoc pooled analysis of the BLISS-52 and BLISS-76 studies to examine the efficacy and safety of belimumab 10 mg/kg IV used in combination with AM, IS and steroids.
Methods
Study design
This post hoc study (GSK study identifier: 201224) was a pooled analysis of efficacy and safety data from patients who received belimumab 10 mg/kg plus SoC compared with placebo (SoC) in the two Phase III, randomized trials BLISS-52 (NCT00424476; GSK study identifier: BEL110752)6 and BLISS-76 (NCT00410384; GSK study identifier: BEL110751).7
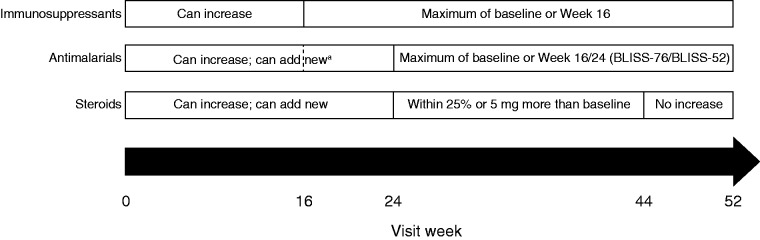
Details of the BLISS-52 and BLISS-76 trials have been described.6,7 Briefly, patients were randomized to receive belimumab 1 mg/kg IV, belimumab 10 mg/kg IV (approved dose) or placebo, plus SoC, for 52 or 76 weeks, respectively. This analysis was based on the belimumab 10 mg/kg and placebo arm data through Week 52. Changes in concomitant medication use were permitted in the BLISS trials (Figure 1). Authors defined the concomitant medication groups using baseline medication data based on their clinical knowledge and experience. Concomitant medication usage at study exit was also described.
Figure 1.
Permitted changes in concomitant medications during the study.
aAntimalarial treatment could be modified up to Week 16 in BLISS-76 and Week 24 in BLISS-52.
BLISS: A Study of Belimumab in Subjects with Systemic Lupus Erythematosus.
Patient population
The primary population was the modified intent-to-treat population, defined as all patients who were randomized and treated with at least one dose of study treatment. Patients were categorized into four baseline concomitant medication groups: steroids only; AM only; steroids + AM; or steroids + AM + IS.
Study endpoints
The primary endpoint was the SRI at Week 52.8 An SRI response was defined as a ≥4-point reduction from baseline in Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) score, no worsening (increase of <0.30 points from baseline) in Physician’s Global Assessment (PGA) and no new British Isles Lupus Assessment Group (BILAG) A organ domain score or two new BILAG B organ domain scores, all compared with baseline at Week 52.
Secondary endpoints included SRI response by visit, ≥4 point reduction from baseline in SELENA-SLEDAI score by visit, change from baseline in PGA by visit and no new BILAG 1A/2B organ domain scores by visit.
Exploratory analyses included time to first SLE flare over 52 weeks (modified SLE Flare Index), change in daily dose of concomitant medication, and SRI for serologically active patients (anti-double-stranded DNA (anti-dsDNA) positive with low complement (C3/C4)) by concomitant medication group.
Safety assessments included treatment-emergent adverse events (AEs), serious AEs (SAEs) and study withdrawals.
Statistical analyses
Results for patient characteristics, efficacy endpoints, AEs, and concomitant medication endpoints are presented as mean (standard deviation) or frequency (percentage), as appropriate. Within each baseline concomitant medication group, Kaplan–Meier estimates of the probability of an SLE flare were calculated and Cox proportional hazards modeling (adjusting for the covariates: baseline SELENA-SLEDAI score, baseline proteinuria level, and race) was used to estimate the hazard ratio (HR) between treatment groups and the corresponding 95% confidence interval (CI). No adjustments for multiple comparisons were performed. Analyses were conducted using SAS® Version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient population
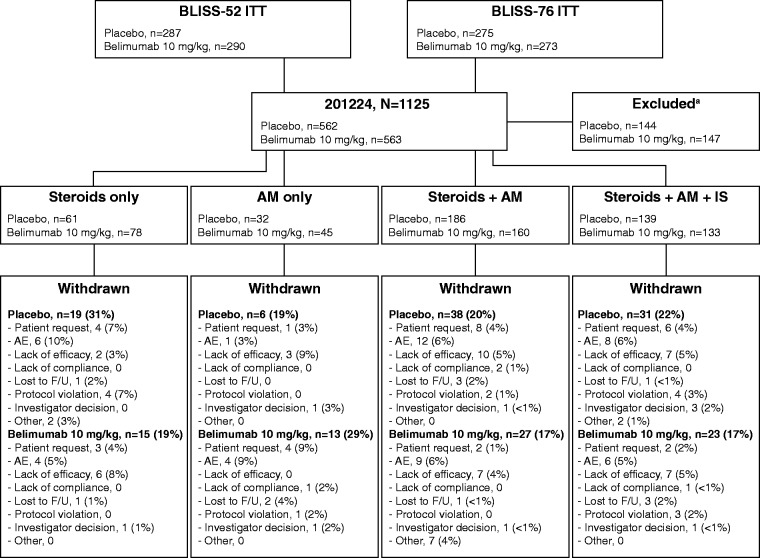
In the BLISS trials, 1125 patients received placebo (n = 562) or belimumab 10 mg/kg (n = 563); 418 and 416 patients, respectively, received concomitant medications at baseline according to the pre-defined groupings and were included in this post hoc analysis. The majority of patients received steroids + AM (n = 346, 41.5%) or steroids + AM + IS (n = 272, 32.6%); the AM-only group contained the fewest patients (n = 77, 9.2%). By Week 52, 94 (22.5%) patients who received placebo and 78 (18.8%) who received belimumab withdrew. The incidence of study withdrawals was comparable between treatment arms in the steroids + AM and steroids + AM + IS groups, lower with belimumab 10 mg/kg (19%) than placebo (31%) in the steroids-only group, and higher with belimumab 10 mg/kg (29%) vs placebo (19%) in the AM-only group. The most common reasons for withdrawal were AEs, lack of efficacy and patient request (Figure 2).
Figure 2.
Patient disposition.
aPatients excluded as they did not receive steroids, AM or IS.
AE: adverse event; AM: antimalarial; BLISS: A Study of Belimumab in Subjects with Systemic Lupus Erythematosus; F/U: follow-up; IS: immunosuppressant; ITT: intent-to-treat.
Baseline patient demographics and characteristics, by concomitant medication group, are shown in Table 1. The percentage of patients randomized to belimumab 10 mg/kg or placebo was balanced within each group. Patients in the steroids + AM or steroids + AM + IS groups were younger than those who received steroids or AM only, and patients in the steroids + AM group had the shortest disease duration. Mean SELENA-SLEDAI scores were similar across groups; a greater proportion of patients receiving belimumab 10 mg/kg in the steroids-only group, and placebo in the steroids + AM + IS group, had a SELENA-SLEDAI score ≥10, compared with other groups. The percentage of patients who were serologically active (i.e. anti-dsDNA positive with low C3/C4) ranged from 33% to 68% across groups and treatment arms.
Table 1.
Patient characteristics at baseline
| Steroids only (N = 139) |
AM only (N = 77) |
Steroids + AM (N = 346) |
Steroids + AM + IS (N = 272) |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Placebo (n = 61, 44%) | Belimumab 10 mg/kg (n = 78, 56%) | Placebo (n = 32, 42%) | Belimumab 10 mg/kg (n = 45, 58%) | Placebo (n = 186, 54%) | Belimumab 10 mg/kg (n = 160, 46%) | Placebo (n = 139, 51%) | Belimumab 10 mg/kg (n = 133, 49%) |
| Female, n (%) | 60 (98) | 73 (94) | 27 (84) | 43 (96) | 174 (94) | 150 (94) | 124 (89) | 132 (>99) |
| Mean age (SD), years | 40.6 (12.11) | 38.4 (11.07) | 42.6 (9.71) | 43.5 (12.37) | 36.1 (11.96) | 36.9 (11.87) | 35.4 (11.47) | 34.4 (9.64) |
| Ethnicity, n (%) | ||||||||
| White/Caucasian | 31 (51) | 32 (41) | 27 (84) | 29 (64) | 75 (40) | 66 (41) | 52 (37) | 52 (39) |
| Asian | 17 (28) | 27 (35) | 1 (3) | 3 (7) | 41 (22) | 41 (26) | 40 (29) | 39 (29) |
| Black/African American | 6 (10) | 7 (9) | 2 (6) | 8 (18) | 17 (9) | 8 (5) | 14 (10) | 14 (11) |
| Other | 7 (11) | 12 (15) | 2 (6) | 5 (11) | 53 (28) | 45 (28) | 33 (24) | 28 (21) |
| Disease duration (SD), years | 7.2 (6.54) | 7.0 (6.51) | 8.4 (7.70) | 6.1 (7.24) | 5.7 (6.16) | 5.7 (6.26) | 6.9 (6.39) | 5.8 (5.77) |
| Mean (SD) SELENA-SLEDAI | 10.3 (4.71) | 10.6 (4.08) | 9.3 (3.65) | 9.6 (3.94) | 9.5 (3.17) | 9.6 (3.85) | 10.2 (4.02) | 9.5 (3.53) |
| SELENA-SLEDAI 6–9, n (%) | 30 (49) | 30 (38) | 19 (59) | 23 (51) | 89 (48) | 80 (50) | 57 (41) | 64 (48) |
| SELENA-SLEDAI ≥10, n (%) | 31 (51) | 48 (62) | 13 (41) | 22 (49) | 97 (52) | 80 (50) | 82 (59) | 69 (52) |
| Mean (SD) PGA | 1.5 (0.48) | 1.5 (0.48) | 1.3 (0.53) | 1.4 (0.43) | 1.4 (0.47) | 1.4 (0.49) | 1.4 (0.48) | 1.3 (0.49) |
| BILAG 1A or 2B, n (%) | 43 (70) | 53 (68) | 22 (69) | 28 (62) | 109 (59) | 90 (56) | 81 (58) | 74 (56) |
| ≥1 severe flare, n (%) | 1 (2) | 2 (3) | 0 | 0 | 0 | 2 (1) | 2 (1) | 1 (<1) |
| Mean (SD) steroid dose, mg/day | 15.4 (8.38) | 15.3 (8.49) | 0 | 0 | 11.4 (7.08) | 11.6 (8.14) | 12.5 (8.88) | 12.8 (9.05) |
| Steroid use >7.5 mg, n (%) | 53 (87) | 66 (85) | 0 | 0 | 117 (63) | 98 (61) | 87 (63) | 87 (65) |
| Anti-dsDNA positive and low C3/C4 | 28 (46) | 49 (63) | 11 (34) | 15 (33) | 114 (61) | 99 (62) | 93 (67) | 91 (68) |
AM: antimalarial; anti-dsDNA: anti-double-stranded DNA; BILAG: British Isles Lupus Assessment Group; C3/C4: complement 3/4; IS: immunosuppressant; PGA: Physician’s Global Assessment; SD: standard deviation; SELENA-SLEDAI” Safety of Estrogen in Lupus National Assessment-Systemic Lupus Erythematosus Disease Activity Index.
Primary endpoint: SRI response at Week 52
At Week 52, the percentage of patients with an SRI response was numerically higher for belimumab 10 mg/kg compared with placebo for all groups (Figure 3). The SRI response treatment difference was greatest in the steroids + AM group (59% vs 44%: odds ratio (OR) 2.04 (95% CI: 1.30–3.20)). A treatment difference was also observed in the steroids-only group (59% vs 49%: OR 1.42 (95% CI: 0.71–2.82)) and steroids + AM + IS group (42% vs 32%; OR 1.65 (95% CI: 0.99–2.74)) The AM-only group had the smallest difference between treatment arms (40% vs 38%: OR 1.10 (95% CI: 0.42–2.91)).
Figure 3.
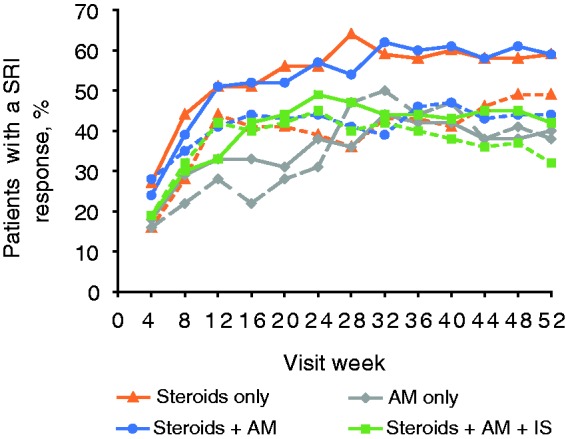
SRI response over time by concomitant medication group.
Solid lines, belimumab 10 mg/kg; dashed lines, placebo.
AM: antimalarial; IS: immunosuppressant; SRI: Systemic Lupus Erythematosus Responder Index.
Secondary endpoints
Numerically more patients who received belimumab 10 mg/kg compared with placebo had an SRI response at Week 12 in the steroids + AM group (51% vs 41%) and in the steroids-only group (51% vs 44%). The AM-only group had the smallest difference at Week 12 (33% vs 28%) and in the steroids + AM + IM group there were fewer responders in the belimumab arm vs placebo (33% vs 42%). At Week 24, a similar pattern was observed across treatment arms, except there were slightly more responders in the belimumab arm vs placebo in the steroid + AM + IS group (49% vs 45%).
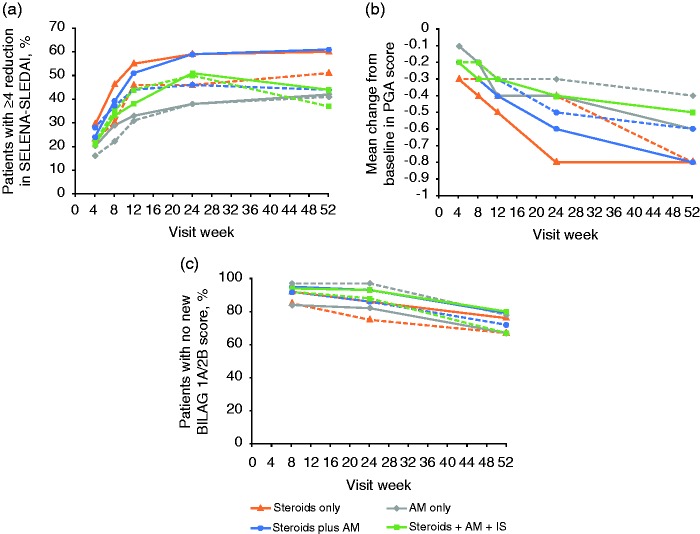
The percentage of patients with a ≥4-point reduction from baseline in SELENA-SLEDAI score was higher for belimumab 10 mg/kg, compared with placebo, in the steroids + AM group at Week 24 and Week 52 (Figure 4(a)). A treatment response was also observed in the steroids-only and steroids + AM + IS groups. At Week 52, there was a greater mean reduction from baseline in PGA score for belimumab 10 mg/kg, compared with placebo, in the AM-only and steroids + AM groups (Figure 4(b)). The percentage of patients with no new BILAG 1A/2B score was greater for those in the steroids + AM + IS group who received belimumab 10 mg/kg, compared with placebo (Figure 4(c)). A treatment response was also observed in the steroids only and steroid + AM groups, but not in the AM-only group.
Figure 4.
Secondary endpoints by visit: (a) Percentage of patients with a ≥4-point reduction in SELENA-SLEDAI. (b) Mean change from baseline in PGA score. (c) Percentage of patients with a new BILAG 1A/2B score.
Solid lines, belimumab 10 mg/kg; dashed lines, placebo.
AM: antimalarial; BILAG, British Isles Lupus Assessment Group; IS: immunosuppressant; PGA: Physician’s Global Assessment; SELENA-SLEDAI: Safety of Estrogen in Lupus National Assessment Systemic Lupus Erythematosus Disease Activity Index.
Time to first flare
By Week 52, the probability of experiencing an SLE flare in the steroids-only group for patients who received belimumab 10 mg/kg compared with placebo was 64.3% vs 78.1% (HR 0.64; 95% CI: 0.42–0.96). In all other groups, the difference between treatment arms in risk of SLE flare was smaller (Table 2).
Table 2.
Time to first flare
| Concomitant medications group | Treatment arm | Probability of an event (95% CI) by Week 52, % | Hazard ratio (95% CI) |
|---|---|---|---|
| Steroids only | Placebo (n = 61) | 78.1 (67.0, 87.6) | 0.64 (0.42, 0.96) |
| Belimumab 10 mg/kg (n = 78) | 64.3 (53.6, 74.9) | ||
| AM only | Placebo (n = 32) | 84.4 (70.0, 94.3) | 0.82 (0.48, 1.38) |
| Belimumab 10 mg/kg (n = 45) | 81.6 (68.3, 91.8) | ||
| Steroids + AM | Placebo (n = 186) | 80.3 (74.2, 85.8) | 0.83 (0.65, 1.06) |
| Belimumab 10 mg/kg (n = 160) | 71.2 (63.9, 78.2) | ||
| Steroids + AM + IS | Placebo (n = 139) | 87.9 (81.7, 92.7) | 0.81 (0.62, 1.05) |
| Belimumab 10 mg/kg (n = 133) | 82.3 (75.3, 88.3) |
AM: antimalarial; CI: confidence interval; IS: immunosuppressant.
Changes in concomitant medications
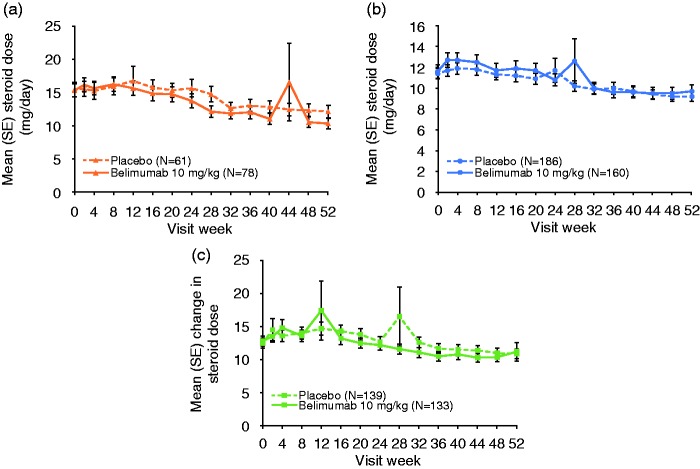
The majority of patients received the same groupings of concomitant medications at the beginning and end of the study (steroids-only group, 93%; AM-only group, 87%; steroids + AM group, 87%; steroids + AM + IS, 87%). For the majority of patients (≥90%) there was no change in AM dose in any group (data not shown). Similarly, for the majority of patients (≥83%) there was no change in IS dose (data not shown). In the AM-only group 15 patients initiated steroids before Week 24 (12 of these were before Week 16) and a further five patients initiated steroids after Week 24; of these five patients, two completed the study and three were withdrawn (protocol violation, investigator decision and lack of efficacy). Mean steroid dose in all other groups fluctuated over time, with a general decrease in dose by Week 52 (Figure 5). The percentage of patients who decreased steroids at each time point increased over time for both treatment arms (Week 52: steroids-only group, 31% placebo, 27% belimumab; steroids + AM group, 22% placebo, 33% belimumab; steroids + AM + IS, 35% placebo, 40% belimumab). In these groups, the percentages of patients with increases in steroid doses at Week 52 were lower than the percentages who reported a decrease (≤11% at Week 52). In the steroids-only group, four patients initiated AM (all before Week 24) and five patients initiated IS before Week 24.
Figure 5.
Mean steroid dose over time by concomitant medication group.
AM: antimalarial; IS: immunosuppressant; SE: standard error.
SRI response in serologically active patients
At Week 52, the percentage of serologically active patients with a SRI response was numerically greater in those treated with belimumab 10 mg/kg compared with placebo in the steroids only group (55% vs 32%), the steroids + AM (60% vs 42%) and steroids + AM + IS groups (42% vs 25%) (Figure 6). The treatment difference was smallest for the AM-only group (40% vs 36%).
Figure 6.
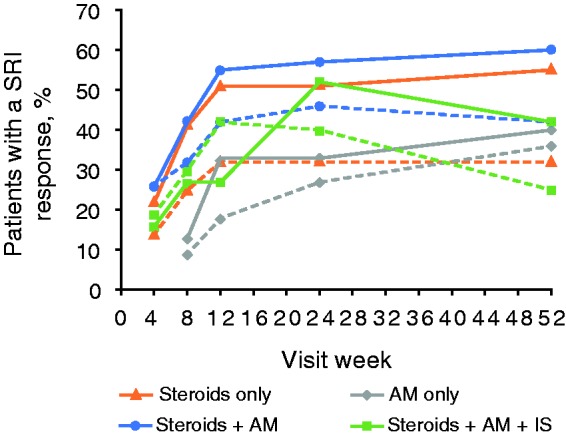
SRI response in serologically active patients (anti-dsDNA positive with low complement).
Solid lines, belimumab 10 mg/kg; dashed lines, placebo.
SRI: Systemic Lupus Erythematosus Responder Index; anti-dsDNA: anti-double-stranded DNA.
Safety
Overall, the incidence of AEs was comparable between treatments within each group (Table 3). The most commonly reported treatment-emergent AEs across all groups (occurring in >10% of patients in either treatment arm) were headache, upper respiratory tract infection, arthralgia, nasopharyngitis, urinary tract infection, diarrhea, nausea and fatigue. Withdrawals due to AEs were consistent across treatment arms and concomitant medication groups.
Table 3.
Adverse events by concomitant medication group
| Steroids only (N = 139) |
AM only (N = 77) |
Steroids + AM (N = 346) |
Steroids + AM + IS (N = 272) |
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 61) | Belimumab 10 mg/kg (n = 78) | Placebo (n = 32) | Belimumab 10 mg/kg (n = 45) | Placebo (n = 186) | Belimumab 10 mg/kg (n = 160) | Placebo (n = 139) | Belimumab 10 mg/kg (n = 133) | |
| Any AEa, n (%) | 56 (92) | 67 (86) | 29 (91) | 41 (91) | 166 (89) | 143 (89) | 133 (96) | 128 (96) |
| Headache | 8 (13) | 10 (13) | 3 (9) | 6 (13) | 47 (25) | 33 (21) | 32 (23) | 30 (23) |
| Upper respiratory tract infection | 10 (16) | 12 (15) | 8 (25) | 10 (22) | 26 (14) | 18 (11) | 35 (25) | 25 (19) |
| Arthralgia | 8 (13) | 10 (13) | 5 (16) | 3 (7) | 17 (9) | 25 (16) | 28 (20) | 20 (15) |
| Nasopharyngitis | 7 (11) | 5 (6) | 1 (3) | 3 (7) | 16 (9) | 11 (7) | 16 (12) | 31 (23) |
| Urinary tract infection | 7 (11) | 11 (14) | 4 (13) | 4 (9) | 17 (9) | 15 (9) | 20 (14) | 18 (14) |
| Diarrhea | 5 (8) | 8 (10) | 5 (16) | 2 (4) | 12 (6) | 11 (7) | 13 (9) | 18 (14) |
| Nausea | 3 (5) | 4 (5) | 3 (9) | 11 (24) | 14 (8) | 14 (9) | 21 (15) | 15 (11) |
| Fatigue | 3 (5) | 4 (5) | 2 (6) | 4 (9) | 10 (5) | 6 (4) | 11 (8) | 17 (13) |
| Bronchitis | 1 (2) | 6 (8) | 3 (9) | 4 (9) | 9 (5) | 12 (8) | 5 (4) | 15 (11) |
| Back pain | 5 (8) | 7 (9) | 3 (9) | 2 (4) | 16 (9) | 12 (8) | 10 (7) | 15 (11) |
| Pyrexia | 8 (13) | 5 (6) | 1 (3) | 4 (9) | 11 (6) | 12 (8) | 13 (9) | 14 (11) |
| Cough | 4 (7) | 4 (5) | 2 (6) | 3 (7) | 13 (7) | 10 (6) | 9 (6) | 14 (11) |
| Insomnia | 3 (5) | 6 (8) | 1 (3) | 3 (7) | 8 (4) | 6 (4) | 7 (5) | 16 (12) |
| Myalgia | 7 (11) | 4 (5) | 2 (6) | 5 (11) | 7 (4) | 7 (4) | 11 (8) | 10 (8) |
| Peripheral edema | 3 (5) | 3 (4) | 2 (6) | 2 (4) | 11 (6) | 8 (5) | 11 (8) | 13 (10) |
| Hypertension | 11 (18) | 3 (4) | 2 (6) | 1 (2) | 15 (8) | 12 (8) | 12 (9) | 8 (6) |
| Sinusitis | 4 (7) | 1 (1) | 4 (13) | 2 (4) | 6 (3) | 7 (4) | 8 (6) | 10 (8) |
| Anemia | 7 (11) | 5 (6) | 1 (3) | 0 | 6 (3) | 7 (4) | 12 (9) | 7 (5) |
| SAE | 13 (21) | 13 (17) | 3 (9) | 4 (9) | 14 (8) | 26 (16) | 29 (21) | 26 (20) |
| AEs leading to withdrawal | 6 (10) | 4 (5) | 1 (3) | 4 (9) | 12 (6) | 9 (6) | 8 (6) | 6 (5) |
AEs occurring in ≥10% of patients in any treatment group are listed.
AE: adverse event; AM: antimalarial; IS: immunosuppressant; SAE: serious adverse event.
The incidence of SAEs was comparable between treatment arms within each group, with the exception of the steroids + AM group (belimumab 10 mg/kg, 16% vs placebo, 8%) (Table 3).
In serologically active patients, the incidence of AEs was comparable to the overall population, and between treatment arms within each group (steroids-only group, 93% placebo, 94% belimumab; AM-only group, 100% both treatment arms; steroids + AM group, 90% placebo, 92% belimumab; and steroids + AM + IS, 97%, both treatment arms). The incidence of SAEs in serologically active patients was also consistent with the overall population.
Discussion
This post hoc analysis from the BLISS trials examined the efficacy and safety of belimumab 10 mg/kg administered with standard SLE care in the following groups: steroids only, AM only, AM + IS, steroids + AM + IS. For all groups, at Week 52, a numerically greater SRI response was observed with belimumab 10 mg/kg compared with placebo; this treatment response was greatest for the AM + steroids group, whereas the AM only group demonstrated the smallest benefit from the addition of belimumab. These data are similar to previous post hoc analyses that suggested patients who received steroids at baseline benefited more from the addition of belimumab than those who did not.9 Across 52 weeks of treatment, SRI response was consistently greater with belimumab 10 mg/kg compared with placebo in the steroids-only group. In the steroids + AM and steroids + AM + IS groups, SRI response was higher with belimumab 10 mg/kg compared with placebo from Week 8 and Week 16, respectively. Overall, the trends in SRI response, individual components of the SRI and time to first flare were similar across the groups, regardless of baseline concomitant medication usage, with the exception of the AM-only group.
The safety profile was similar between belimumab and placebo for all groups, with the exception of SAEs in the steroids + AM group, which were higher with belimumab compared with placebo. The rate of withdrawals was higher in the belimumab 10 mg/kg group compared with placebo for patients receiving AM alone. These withdrawals were largely due to patient request or as a result of AEs, and are consistent with the efficacy results where the AM-only group demonstrated the smallest benefit from the addition of belimumab.
Changes in concomitant medications were subject to BLISS protocol restrictions. Despite the lack of a protocol-mandated steroid taper and protocol-expressed caution on steroid tapering, the percentage of patients who decreased steroids at each time point increased over time for both treatment arms. The SLE treatment patterns revealed in this analysis can be considered in the context of the real-world treatment patterns. A retrospective, observational cohort study conducted in United States (US) patients receiving belimumab for the treatment of SLE reported that 94% of patients used a standard SLE therapy (corticosteroid, IS, AM) at some time during the six-month pre-index and six-month post-index period of the study, with a general decrease in steroid use observed in the post-index period.10 Similarly, in the OBSErve US study of patients receiving belimumab, the mean steroid dose and the percentage of patients who received steroids decreased over 24 months.11
Several studies have reported sustained efficacy of belimumab administered in combination with steroids, with many patients being able to reduce or even discontinue steroid use over time, suggesting a potential role for belimumab as a steroid-sparing agent, which requires further investigation.12–14 The concomitant medications included in this study are representative of those described in patients with SLE where steroids, AM and IS have been among the preferred treatments.15
There are several limitations to the interpretation of these results. In the BLISS trials, patients were not randomized by concomitant medications, therefore patient numbers varied between the groups examined and the study was not powered to investigate differences between these groups. The low number of patients in the AM-only group (n = 77) limits the conclusions that can be drawn from these results, and the authors feel that discussion of statistical significance is not relevant on this basis. Concomitant medication groups were determined using baseline data; interestingly, few patients altered treatments across the year. The BLISS trials restricted when and how concomitant medications could be altered, therefore changes in medication and dosages may not be representative of real-world clinical practice. Finally, the BLISS trials did not collect concomitant medication history prior to study participation. It would be of interest to investigate what treatment histories patients had prior to study enrollment.
In summary, the efficacy of belimumab in combination with SoC was demonstrated for steroids, alone and in combination with AM and IS. With the exception of SAEs in the steroids + AM group, the safety profile was similar across all medication groups and treatment arms. These findings may be helpful in understanding current prescribing practice trends and may improve the understanding of the safety and efficacy of adding belimumab to various treatments.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A Schwarting: consulting fees from GSK and Actelion. MA Dooley: consulting fees from GSK, Genentech and Biogen IDEC Inc, EMD Serono, Medimmune, and UCB, and has other interests in Lilly and Aurinia. DA Roth: shareholder of GSK, employee of GSK. L Edwards: shareholder of GSK, employee of Parexel. A Thompson: shareholder of GSK, employee of GSK. B Wilson: shareholder of GSK, former employee of GSK.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by GSK. Medical writing assistance was provided by Louisa Pettinger, PhD, of Fishawack Indicia Ltd, and was funded by GSK.
References
- 1.Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis 2006; 1: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. New Eng J Med 2008; 358: 929–939. [DOI] [PubMed] [Google Scholar]
- 3.GlaxoSmithKline. Benlysta Prescribing Information, https://www.gsksource.com/gskprm/htdocs/documents/BENLYSTA-PI-MG.PDF (2015, accessed 5 November 2014).
- 4.Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 2003; 48: 3253–3265. [DOI] [PubMed] [Google Scholar]
- 5.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 2009; 119: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 7.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furie RA, Petri MA, Wallace DJ, et al. Novel evidence-based Systemic Lupus Erythematosus Responder Index. Arthritis Rheum 2009; 61: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: High disease activity predictors of response. Ann Rheum Dis 2012; 71: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xe K, Eisenberg DF, Oglesby A, Patel J, Kan H, Boggs R. A retrospective administrative claims database evaluation of the utilization of belimumab in US managed care settings. Clin Ther 2015; 37: 2852–2863. [DOI] [PubMed] [Google Scholar]
- 11.Collins CE, Dall’Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med 2016; 3: e000118–e000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreoli L, Reggia R, Pea L, et al. Belimumab for the treatment of refractory systemic lupus erythematosus: Real-life experience in the first year of use in 18 Italian patients. Isr Med Assoc J 2014; 16: 651–653. [PubMed] [Google Scholar]
- 13.Ginzler EM, Wallace DJ, Merrill JT, et al. Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. J Rheum 2014; 41: 300–309. [DOI] [PubMed] [Google Scholar]
- 14.Hui-Yuen JS, Li XQ, Askanase AD. Belimumab in systemic lupus erythematosus: A perspective review. Ther Adv Musculoskelet Dis 2015; 7: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muangchan C, van Vollenhoven RF, Bernatsky SR, et al. Treatment algorithms in systemic lupus erythematosus. Arthritis Care Res 2015; 67: 1237–1245. [DOI] [PubMed] [Google Scholar]