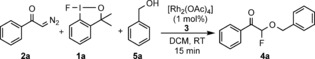
Table 1.
Oxyfluorination of 2 a under various reaction conditions.[a]
| Entry | Deviation from the optimal reaction conditions | Yield [%][b] |
|---|---|---|
| 1 | none | 62 |
| 2 | 1.2 equiv of 2 a | 50 |
| 3 | 5 mol % of 3 | 65 |
| 4 | [Rh(PPh3)3Cl] | 5[c] |
| 5 | [{Rh(COD)Cl}2] | 6[c] |
| 6 | [Rh2(esp)2] | 56 |
| 7 | without 3 | NR |
| 8 | −20 °C instead of RT | 60 |
| 9 | THF | 32 |
| 10 | toluene | 47 |
| 11 | acetonitrile | 44 |
| 12 | Selectfluor instead of 1 a | 0 |
| 13 | NFSI instead of 1 a | 60 |
[a] Optimal reaction conditions: 1 a (0.1 mmol), 2 a (0.2 mmol), 5 a (0.3 mmol), [Rh2(OAc)4] (3; 0.001 mmol) was reacted in DCM (2.0 mL) for 15 minutes at RT. [b] Yield of isolated product. [c] 19F NMR yield using trifluoromethyl benzene as internal standard. COD=1,5‐cyclooctadiene, DCM=dichloromethane, NFSI=N‐fluorobenzenesulfonimide, THF=tetrahydrofuran.
