Figure 6.
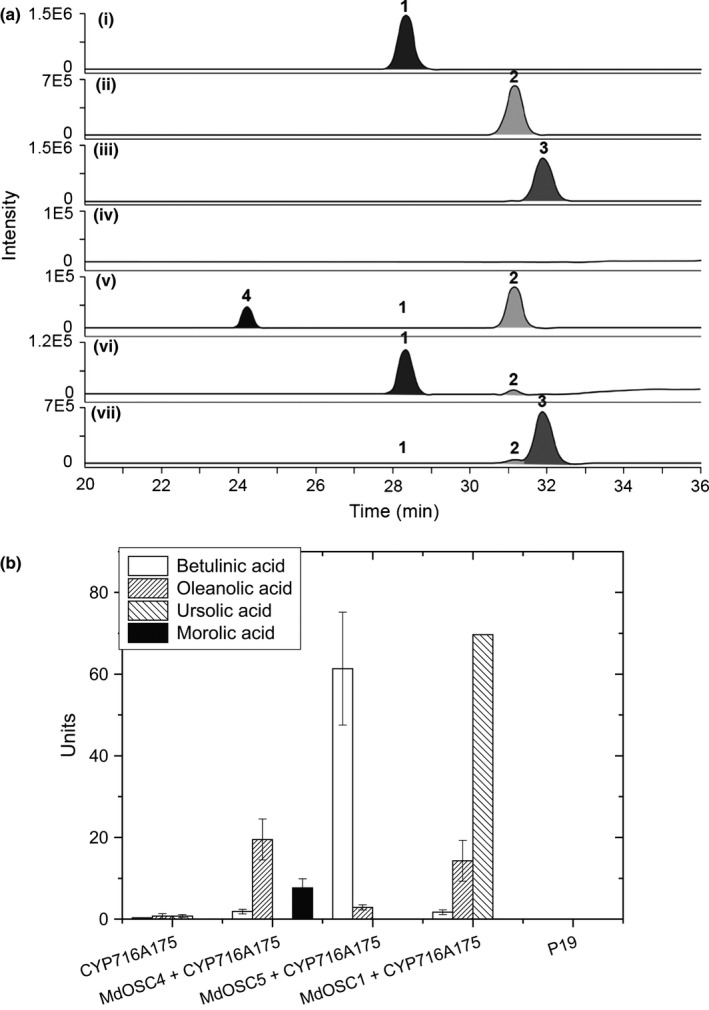
Triterpene acid synthesis in tobacco leaves by heterologous coexpression of a cytochrome P450 (CYP) gene with triterpene synthases. (a) Chromatograms of typical LC‐MS traces of the products of CYP716A175 (P450) coexpressed with (v) Malus × domestica OXIDOSQUALENE CYCLASE4 (MdOSC4), (vi) MdOSC5, and (vii) MdOSC1. p19 was used as a negative control (iv) and compared with authentic standards of (i) betulinic acid (1) at 100 μg ml−1; (ii) oleanolic acid (2) at 100 μg ml−1, and (iii) ursolic acid (3) at 100 μg ml−1. (4) Putative identification of morolic acid. Compounds were identified and quantified on the basis of their mass spectral data (Supporting Information Fig. S6). Chromatograms are presented as selected ion plots of the sum of three ions: m/z 474.6 [M+NH 4]+, m/z 456.6 [M]+ and m/z 439.8 [MH‐H2O]+. (b) Quantification of the amount of triterpene acids made by the addition of a P450 gene to the triterpene synthases. Morolic acid was quantified as oleanolic acid equivalent. Data are nmoles of triterpenes produced from 100 mg of extracted tissue (mean ± SE; n = 4).
