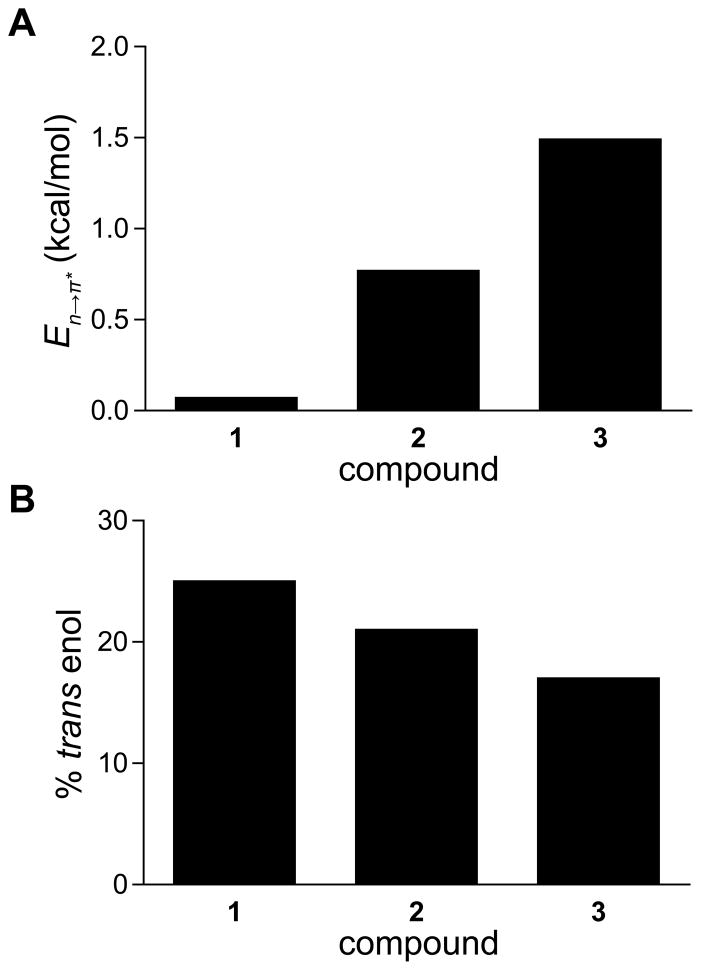
Figure 4.
(A) Energies of n→π* interactions in β-keto amides 1–3, as calculated in vacuo from second-order perturbation theory implemented by NBO 5.9.20 (B) Percentage of β-keto amides 1–3 populating the trans enol form in CDCl3 at 25 °C, as determined by 1H NMR spectroscopy. Repeated integration yields values within 1% of those depicted.