Figure 1.
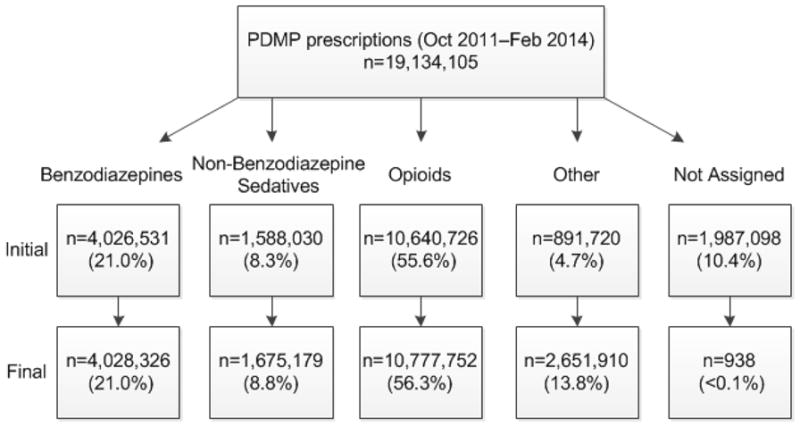
PDMP drug category methodology showing initial and final distribution of PDMP prescriptions within each drug category.
Drug category methodology showing initial and final distribution of prescription drug monitoring program prescriptions within each drug category. Drug categories were assigned using National Drug Code drug reference table definitions. The drug product, alone or in combination with other chemicals, included substances in the following pharmaceutical classes: Benzodiazepines, Non-benzodiazepine sedatives (e.g., zaleplon, zolpidem, or barbiturates), Opioids (e.g. opioid agonists, partial opioid agonists), Other: schedule II stimulants (8.2%), pseudoephedrine (1.8%) schedule III-IV appetite suppressants (1%), the muscle relaxant carisoprodol (<1%), and other scheduled medications not otherwise categorized (1.9%).
