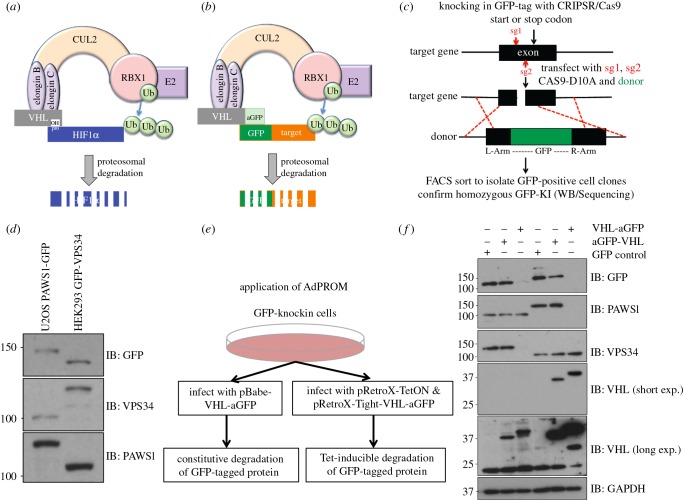
Figure 1.
The affinity-directed protein missile (AdPROM) system degrades target proteins in different cell lines. (a) Schematic describes how the CUL2-E3 ligase complex results in ubiquitylation and degradation of its native target, hydroxy-proline modified HIF1α, under normoxic conditions. (b) Schematic of the exploitation of the CUL2 E3 ligase machinery for AdPROM using anti-GFP nanobody (aGFP) to degrade GFP-tagged proteins. (c) Strategy for rapidly knocking in a GFP tag on target proteins in somatic cells using CRISPR/Cas9. (d) Western blot analysis of extracts from PAWS1-GFP and GFP-VPS34 knockin U2OS and HEK293 cells, respectively, using the indicated antibodies. (e) Schematic shows the application of AdPROM using pBABED-Puro (for constitutive expression) or pRetroX-TetON (for Tet-inducible expression) retroviral infection systems to introduce the VHL-aGFP in GFP-knockin cells. (f) A proof-of-principle demonstration of the efficacy of the AdPROM system in the degradation of GFP-VPS34 and PAWS1-GFP from knockin HEK293 and U2OS cells, respectively. Cells infected with control retroviruses (GFP) or retroviruses encoding aGFP-VHL or VHL-aGFP were lysed. Extracts (20 µg protein) were subjected to resolution by SDS–PAGE and transferred to PVDF membranes, which were analysed by western blotting with the indicated antibodies.