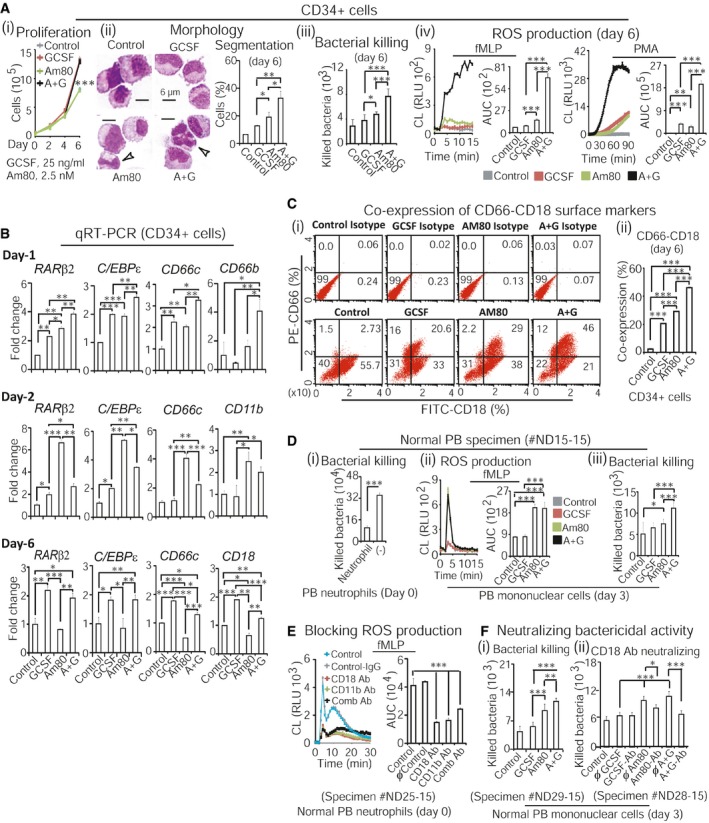
Proliferation analysis of hematopoietic CD34+ precursors for up to 6 days (i). Neutrophil morphologic differentiation, bacterial killing, and ROS production were assessed at day 6 (ii–iv). Controls were without treatment. White arrows indicate neutrophil nuclear segmentation.
qRT–PCR‐assessed RA‐target gene expression after culturing CD34+ cells for 1, 2, and 6 days.
Flow cytometry analysis of CD66‐CD18 co‐expression after culturing CD34+ cells for 6 days, using anti‐human CD66‐PE and CD18‐FITC antibodies (i). Isotypes were used for controls (i, top panel). CD66‐CD18 co‐expression was quantified in (ii).
Fresh peripheral blood (PB) was collected from normal human donor. Bacterial killing was assessed in the presence or absence of PB neutrophils (i). Bactericidal activities of neutrophils induced by Am80‐GCSF from PB mononuclear cells were evaluated by ROS production (ii) and bacterial killing assays (iii).
Fresh PB neutrophils collected from normal human donor were tested for ROS production in the presence or absence of specific antibodies. Controls were with or without IgG. Comb Ab, combined anti‐CD18 and ‐CD11b antibodies.
Fresh PB mononuclear cells collected from normal human donors were treated for 3 days. Bacterial killing (i) and neutralization of bacterial killing in the presence of IgG or anti‐CD18 antibody were assessed at day 3 (ii).
Data information: Data are shown as mean ± SD and represent at least two independent experiments with similar results. *
P <
0.05; **
P <
0.01; ***
P <
0.001 (Student's unpaired two‐tailed
t‐test). Exact
P‐values are provided in
Appendix Table S4. A+G, Am80‐GCSF combination; CL, chemiluminescence; RLU, relative light units; AUC, area under the curve; ϕ, normal IgG; Ab, antibodies.