Figure 9. Model for the movement of SNARE TMDs during vacuole fusion.
-
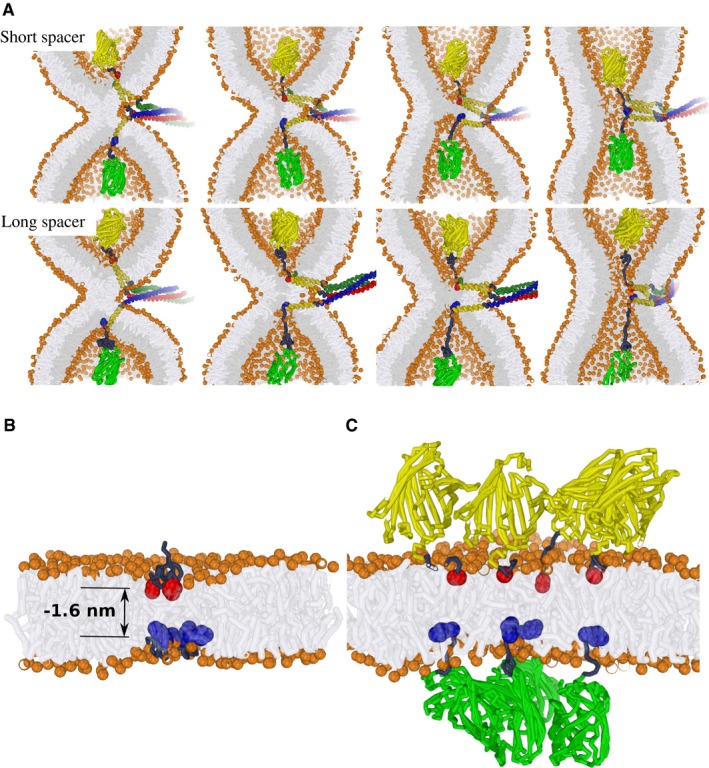
AExamples of simulated hemifusion‐to‐fusion transitions in the presence of modified vacuolar SNARE complexes. Upper panel: Short spacer. Lower panel: Long spacer. These stirred molecular dynamics simulations illustrate that the progression of fusion is sterically achievable for both of the fluorescent protein‐tagged SNARE complexes, even when the geometry of the fusion zone is extremely confined, that is, the fusion between two highly curved membrane dimples (1/8 nm−1). This scenario suggests that the fusion‐inhibiting effect of a fluorescent protein added via a short peptide spacer is unlikely to result from a direct “steric clash” with the membrane. Vam3 in red/yellow; Nyv1 in blue/yellow; Vti1/Vam7 in dark green and yellow; bulky fluorescent protein tags in light green and yellow, respectively.
-
B, CA scenario simulating the presence of four SNARE complexes near the fusion site. The modeled indentation corresponds to a relative indentation of −1.6 nm (see Fig 2B). This corresponds to an exaggerated indentation force of about 80 pN which we exploit to demonstrate the clustering between “indentations” within our limited simulation time. The C‐terminal amino acid of each Vam3 and Nyv1 molecule is represented by a red or blue bead, respectively; bulky fluorescent protein tags in light green and yellow, respectively. (B) Isolated SNARE C‐termini with short peptide tag. The C‐termini concentrate in a small zone (similar membrane perturbations attract each other). (C) Steric hindrance by the addition of bulky proteins prevents collective, localized force transduction by multiple SNARE complexes.
