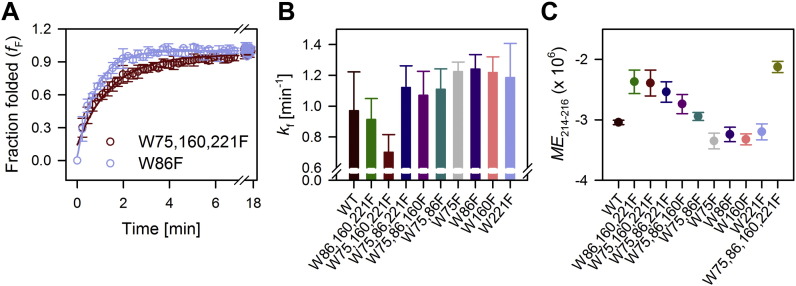
Fig. 3.
Folding rates and secondary structure content of hVDAC-2 WT and various tryptophan mutants. (A) Folding kinetics of hVDAC-2 W75,160,221F and W86F monitored using tryptophan fluorescence anisotropy in 19.5 mM DDM at 4 °C, highlights the difference in the rates between the two mutants. Solid lines indicate fits to a single exponential function. The folding kinetics for all the mutants and the raw anisotropy data is shown in Supplementary Fig. 2. (B) Histograms summarizing the rates obtained from (A), after fitting individual data to a single exponential function. hVDAC-2 W75,160,221F shows the slowest folding rate in DDM micelles. The initial dead time of the experiment was ~ 25 s, and the initial folding phase could not be accurately captured. Error bars specify standard deviation from three independent experiments. (C) ME214–216 shows an average of molar ellipticity (ME) values from 214–216 nm, and is used as an indicator of the secondary structure content in folded hVDAC-2. W75,86,160,221F (Trp-less) and all the single tryptophan mutants show a compromised folding efficiency in DDM micelles, and therefore, have lower ME values. Put together, these data highlight the importance of tryptophans for proper folding of the hVDAC-2 barrel.