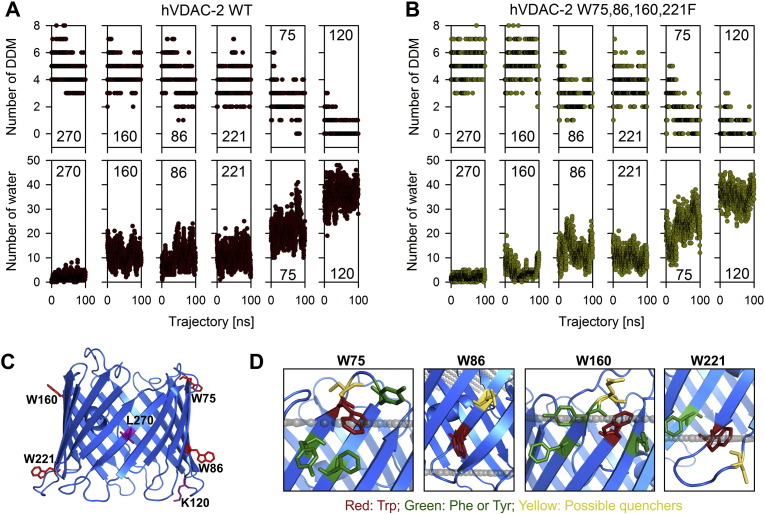
Fig. 8.
Vicinity analysis of Trp/Phe from simulation studies. Analysis of Trp or Phe at positions 75, 86, 160, 221, and control residues (120 and 270) in a 100 ns simulation of hVDAC-2 WT and W75,86,160,221F in DDM micelles. DDM/water molecules within a 5 Å radius of the designated residues throughout the trajectory are shown for WT (A) and W75,86,160,221F (B) barrels. Lys-120 and Leu-270 served as controls for water-solvated and DDM-bound regions, respectively. The residue number is indicated within each panel. Comparing the total number of neighboring DDM (upper panels) and water molecules (lower panels) within a 5 Å radius of each tryptophan shows that 160th position has high DDM content, comparable to that of the completely buried Leu-270 residue. This highlights the affinity of Trp-160 for a hydrophobic environment. At the same time, this position has a similar water content as the other tryptophans (75, 86, 221; see lower panels), reflecting the interfacial nature of this region. Similar results were obtained for WT and W75,86,160,221F simulations. Also see Supplementary Fig. 10 for more information. (C) Cartoon representation of hVDAC-2 WT barrel showing the positions of four intrinsic tryptophans (red sticks) and the control residues, K120 and L270 (purple sticks). (D) Cartoon representation of hVDAC-2 WT highlighting the neighboring aromatic residues (green sticks), and possible quenchers (yellow sticks), for each of the four tryptophans (red sticks). Possible quenchers for tryptophan fluorescence include residues like cysteine, histidine, glutamic acid and aspartic acid. The membrane position is shown as grey spheres.