Figure 10.
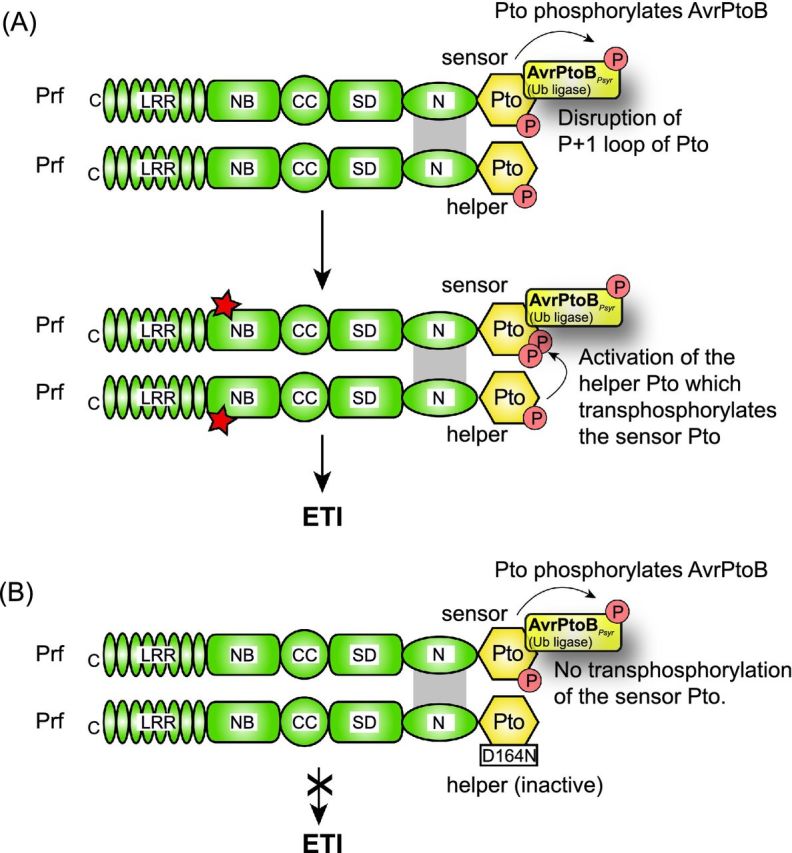
Model of Prf/Pto-triggered activation of plant defense responses. (A) AvrPtoB triggers Prf/Pto-dependent ETI responses. The CC-NB-LRR protein Prf contains an SD (Solanaceae domain) domain with weak homology to other solanaceous R proteins and an N-terminal domain (N), which interacts with the Pto kinase and mediates the Pto-independent dimerization of Prf (Gutierrez et al.2010). The Prf/Pto complex contains at least two molecules of each protein. Pto is autophosphorylated at S198 and required to maintain Prf in an inactive conformation. The sensor Pto molecule interacts with and phosphorylates the E3 ubiquitin ligase AvrPtoB from P. syringae and evades AvrPtoB-mediated degradation. Effector binding to the P+1 loop of the Pto sensor presumably triggers a conformational change in this loop, which activates the Pto helper protein. The Pto helper molecule transphosphorylates the sensor at amino acid residue T199. Transphosphorylation of the sensor Pto leads to the activation of Prf (indicated by a red asterisk) and thus to Prf/Pto-triggered ETI responses. (B) A kinase-inactive Pto helper molecule (PtoD164N) does not transphosphorylate the kinase-active Pto sensor and therefore does not activate Prf/Pto-dependent ETI responses.
