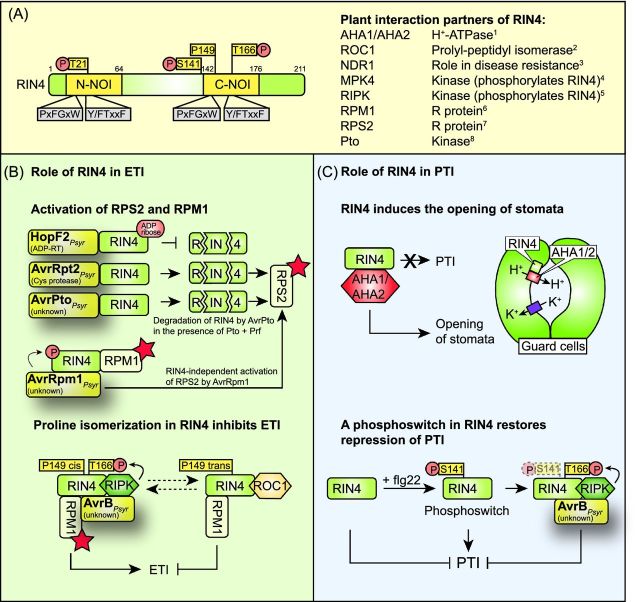
Figure 11.
Effector-triggered modifications of RIN4 and their contributions to PTI and ETI responses. (A) Domain organization of RIN4 and list of known plant interaction partners of RIN4. RIN4 contains N- and C-terminal NOI domains with conserved PxFGxW and Y/FTxxF amino acid motifs. The PxFGxW motif is the cleavage site of the effector protein AvrRpt2 from P. syringae. The Y/FTxxF motif contains a conserved threonine residue, which is phosphorylated by the effector proteins AvrB and AvrRpm1 from P. syringae. Additional important amino acids are indicated (see the text for details). Known plant interaction partners of RIN4 and their predicted functions are listed. References: 1, Liu et al. (2009); 2, Li et al. (2014b); 3, Day, Dahlbeck and Staskawicz (2006); 4, Cui et al. (2010); 5, Liu et al. (2011); 6, Mackey et al. (2002); 7, Axtell and Staskawicz (2003); 8, Luo et al. (2009). (B) Contribution of RIN4 to RPS2- and RPM1-triggered ETI responses. The ADP-RT HopF2 from P. syringae ADP-ribosylates RIN4 and suppresses the degradation of RIN4 by the cysteine protease AvrRpt2 from P. syringae. RIN4 is also degraded by AvrPto from P. syringae in the presence of Pto and Prf. The cleavage products of RIN4 are detected by the R protein RPS2, which triggers ETI. The effector proteins AvrRpm1 and AvrB from P. syringae lead to the phosphorylation of RIN4 and thus to the activation of the R protein RPM1 (indicated by a red asterisk). Effector-triggered phosphorylation of RIN4 presumably depends on the kinase RIPK, which interacts with RIN4 and AvrB and phosphorylates RIN4 at several amino acid residues including T166. The phosphorylation of T166 of RIN4 interferes with the interaction of RIN4 with the cyclophilin ROC1, which catalyzes the cis/trans isomerization of the proline residue at position 149 of RIN4. The cis/trans isomerization of P149 presumably leads to a specific conformational change in RIN4 and thus inhibits the activation of RPM1 and RPS2. Phosphorylation of RIN4 in the presence of AvrB likely induces conformational changes, which interfere with ROC1-mediated isomerization of RIN4 and lead to the activation of RPM1 (see the text for details). (C) Model of the role of RIN4 during PTI. RIN4 is a suppressor of PTI responses and interacts with and activates the H+ ATPases AHA1 and AHA2, which pump protons from the cytosol of guard cells into the apoplast. The activity of AHA1/2 leads to the establishment of a proton electrochemical gradient, which is used by channel and carrier proteins to mediate the uptake of ions into guard cells, thus leading to stomatal opening. Upon detection of flg22 during PTI, RIN4 is phosphorylated at amino acid S141, which leads to the derepression (= activation) of PTI. The phosphorylation of RIN4 at amino acid T166 by AvrB and AvrRpm1 from P. syringae presumably counteracts the effect of the S141 phosphorylation and restores the repression of PTI (see the text for details).