Table 1.
Optimization of cyclooctane fluorination
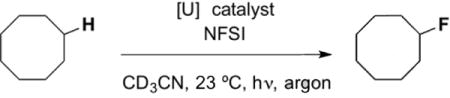
| ||||
|---|---|---|---|---|
| Entry | NFSI [equiv.] | Catalyst [mol%] | Light Source[a] | Yield [%][b] |
| 1 | 1.5 | UO2(OAc)2•4H2O (1) | A | 8 |
| 2 | 1.5 | UO2(NO3)2•6H2O (1) | A | 52 |
| 3 | 1.5 | UO2(NO3)2•6H2O (1) | B | >95 |
| 4 | 1.5 | UO2(NO3)2•6H2O (1) | none | not observed |
| 5 | 1.5 | none | B | not observed |
| 6 | 1.2 | UO2(NO3)2•6H2O (1) | B | 73 |
| 7 | 1.5 | UO2(NO3)2•6H2O (0.5) | B | 52 |
| 8[c] | 1.5 | UO2(NO3)2•6H2O (1) | B | >95 |
Light source A: High Density blue LED strip; Light source B: high intensity blue LED lamp
Yield determined by NMR through integration relative to a methyl acetate internal standard
Acetone-d6 used as solvent
