Fig. 2. Peptides PCAIWF and WVCSGG interact with the ligand-binding domain of VEGFR-3.
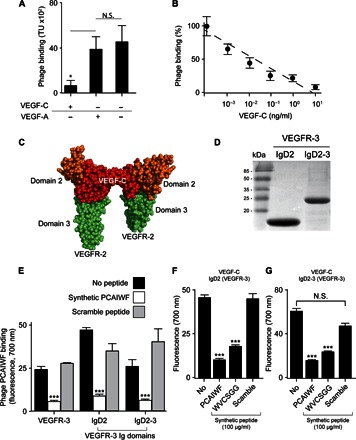
(A) Binding of phage PCAIWF to immobilized VEGFR-3 in the presence or absence of VEGF-A or VEGF-C (10 ng/ml). (B) Binding of phage PCAIWF to immobilized VEGFR-3 in the presence of increasing concentrations of VEGF-C. Percentage relative to phage binding in the absence of VEGF-C. (C) Cartoon showing the three-dimensional structure of the complex VEGF-C (red) bound to VEGFR-2 IgD2-3 (shown in orange and green, respectively) (Protein Data Bank #2X1W). (D) Analysis by SDS–polyacrylamide gel electrophoresis of purified recombinant IgD2 and IgD2-3 proteins containing the ligand-binding domain of VEGFR-3. (E) Binding of phage PCAIWF to VEGFR-3 and its recombinant Ig domains immobilized on microtiter wells in the presence or absence of the synthetic peptide PCAIWF or its scramble version, IFCAPW (100 μg/ml). Phage binding was quantified by FLISA using an anti-bacteriophage sera. (F) Binding of VEGF-C to microtiter wells coated with immobilized recombinant ligand binding domains IgD2 and IgD2-3 of VEGFR-3 in the presence or absence of synthetic peptides PCAIWF and WVCSGG or the scramble control peptide (IFCAPW). For phage experiments (A and B), bars represent mean ± SEM from triplicate plating; for FLISA assays (E to G), bars represent means ± SEM from duplicate wells. Statistics, Student’s t test [not significant (N.S.), P > 0.05; *P ≤ 0.05 and ***P ≤ 0.001].
