Abstract
Background. Antibodies to the cysteine-rich domain II of Plasmodium vivax Duffy binding protein (PvDBP) can inhibit binding of this parasite ligand to its receptor on red blood cells, the Duffy antigen/receptor for chemokines. These binding-inhibitory antibodies (BIAbs) also inhibit P. vivax invasion of reticulocytes in vitro.
Methods. To investigate whether naturally acquired anti-PvDBP antibodies are associated with reduced risk of clinical malaria in a population exposed to low levels of P. vivax transmission, we measured total levels of immunoglobulin G antibodies to 5 PvDBP variants and used a functional in vitro assay to quantify their binding-inhibitory activity in a cohort of 466 rural Amazonians followed up for up to 37 months.
Results. No association between total immunoglobulin G antibody responses to any PvDBP variant and risk of symptomatic, laboratory-confirmed vivax malaria was observed in this cohort. However, a Cox proportional hazards model, adjusted for age, sex, and genotype for the Duffy antigen/receptor for chemokines, showed a >40% decrease in the prospective risk of clinical vivax malaria in subjects with the strongest BIAb responses (upper and middle terciles). High BIAb responses were mostly PvDBP variant transcending and stable over time.
Conclusions. Strong naturally acquired BIAb responses are associated with a reduced risk of clinical P. vivax malaria in rural Amazonians.
Keywords: malaria, antibodies, Plasmodium vivax, Duffy binding protein, Duffy antigen/receptor for chemokines, immunity, Amazon
Clinical manifestations of Plasmodium vivax malaria are caused by asexual parasites multiplying inside red blood cells (RBCs). Children exposed to intense transmission experience repeated P. vivax malaria attacks during their first years of life, whereas adolescents and adults may harbor low-grade infections but usually remain free of symptoms once parasitized [1]. Naturally acquired immunity does not prevent infection but limits parasite multiplication and reduces the proportion of infections that progress to disease [2]. Clinical immunity also develops under the conditions of substantially lower P. vivax transmission that prevail in the Amazon. Although malaria episodes are experienced by children and adults, an increased proportion of infections remain asymptomatic in subjects with >5–8 years of continuous exposure to infection [3–5].
Several receptor-ligand interactions are required for P. vivax invasion of RBCs. A key step involves the cysteine-rich domain II of the P. vivax Duffy binding protein (PvDBP) and its cognate receptor on RBCs, the Duffy antigen/receptor for chemokines (DARC). PvDBP binding to DARC is needed to form an irreversible junction between invading P. vivax merozoites and their host cells, immature RBCs known as reticulocytes [6]. RBCs lacking DARC are typically refractory to P. vivax infection [7].
Strong naturally acquired antibody responses to PvDBP are associated with reduced prospective risk of high-density parasitemia in Papua New Guinean children exposed to intense P. vivax transmission [8]. Children with high levels of binding-inhibitory antibodies (BIAbs), which are able to inhibit PvDBP binding to DARC in vitro, are protected from subsequent P. vivax infections detected with microscopy [9]. Moreover, human anti-PvDBP antibodies purified from the serum of these children, as well as anti- Duffy binding protein antibodies elicited in immunized rabbits, can inhibit P. vivax invasion of reticulocytes in vitro [10].
To investigate whether naturally acquired anti-PvDBP antibodies reduce the risk of clinical malaria in a population exposed to low-level P. vivax transmission, we measured levels of immunoglobulin (Ig) G antibodies to 5 PvDBP variants in a cohort of rural Amazonians and used a functional assay to quantify their binding-inhibitory activity. We show that high levels of BIAbs, but not of total anti-PvDBP IgG antibodies, persist for several months, are variant transcending, and confer protection from subsequent clinical vivax malaria in this population.
METHODS
Cross-sectional Surveys
Between March 2010 and May 2013, 7 cross-sectional surveys were carried out in farming settlements scattered in the equatorial rain forest of Remansinho area, northwestern Brazil (Supplementary Figure 1). All inhabitants older than 3 months were invited to contribute 5-mL venous blood samples, irrespective of any clinical symptoms, for malaria diagnosis, antibody assays, and Duffy blood group genotyping [11]. Study protocols were approved by the National Human Research Ethics Committee of the Ministry of Health of Brazil (approval No. 551/2010). Written informed consent was obtained from all study participants or their parents or guardians.
Malaria Diagnosis and Surveillance
Conventional microscopy of Giemsa-stained thick smears and quantitative polymerase chain reaction (qPCR) were used to diagnose malaria in cross-sectional surveys [11]. At least 100 fields were examined for malaria parasites by 2 experienced microscopists, under ×1000 magnification, before slides were declared negative. Species-specific primers were used to amplify a 100–base pair fragment of the 18S ribosomal RNA genes of P. vivax and Plasmodium. falciparum by real-time qPCR, with a detection threshold of 2 parasites/microliter of blood [12]. To estimate the incidence of clinical malaria over the study period, trained local health workers carried out weekly house-to-house visits from March 2010 to October 2013. Thick smears were systematically obtained from subjects with fever, chills, headache, or any other malaria-related symptoms and or signs at the time of blood collection or those reporting these symptoms within the past 48 hours. Slide-positive subjects were treated according to the current malaria therapy guidelines of the Ministry of Health of Brazil [13].
DARC Genotyping
TaqMan assays [14] were used to genotype 2 DARC polymorphisms: the T-33C substitution in the RBC-specific GATA1 transcription factor binding motif (rs2814778), which suppresses DARC expression on RBC surface (FY*BES allele), and the G125A polymorphism (rs12075), which defines the FY*B (wild type) and FY*A (mutated) alleles.
Recombinant Antigens
We measured IgG antibodies to the following: (1) 5 variants (Sal I, AH, P, C, and O [8]) of the domain II (residues 194–521) of PvDBP [15]; (2) the C-terminal, 19-kDa region of P. vivax merozoite surface protein (MSP) 1 (PvMSP-119), Belém strain, kindly provided by Anthony Stowers (National Institute of Allergy and Infectious Diseases, National Institutes of Health); (3) block II (nucleotides 1246–2058) of P. vivax MSP-3α (PvMSP-3α) from the Belém strain [16]; (4) the N-terminal region (residues 34–193) of P. vivax MSP-9 (PvMSP-9) from the Belém strain [17], kindly provided by Mary Galinski (Emory University); and (5) the F2 region of P. falciparum erythrocyte-binding antigen (PfEBA) 175, kindly provided by Chetan Chitnis (International Centre for Genetic Engineering and Biotechnology).
Bead-Based Multiplex Assay for Antibodies
Spectrally unique carboxylated xMAP microspheres (Luminex) coupled to recombinant proteins were used to detect antigen-specific total IgG antibodies [18]. Briefly, 1000 coupled beads per well for each antigen were incubated with patients' plasma at a 1:200 dilution in 96-well microplates (MultiScreenBV MSBVN1210; Millipore). R-phycoerythrin–conjugated, anti-human IgG (Jackson Immunoresearch) was used to detect antibody binding. Serially diluted plasma samples from a pool of infected adults from Papua New Guinea were used as positive controls, starting at 1:50 dilution, and plasma samples from 20 North Americans who had not been exposed to malaria served as negative controls. (We did not use samples from Amazonians as negative controls because we would be unable to rule out past exposure to malaria.) We read fluorescence from 75 beads per antigen on Bio-Plex 200 equipment with Bioplex Manger 6.1 software (Bio-Rad). The mean fluorescence intensity (MFI) obtained with negative controls for each antigen, plus 3 standard deviations, was used as the cutoff value to define a positive response. Quantitative results were expressed as reactivity indices, calculated as the sample MFI divided by the cutoff MFI value; an index >1 was considered positive.
Microplate-Based Binding Inhibition Assay
Functional assays were performed in duplicate to test plasma samples for the presence of BIAbs [19]. Plasma samples were preincubated at 1:20 dilution with the recombinant Sal I variant of PvDBP (0.011 µg/mL; kindly provided by Niraj Tolia, Washington University School of Medicine) and transferred to Immulon 2B microplates (ThermoScientific) coated (at 12.5 ng per well) with the N-terminal region of the human DARC protein ligated to the Fc region of human IgG (nDARC-Fc). Bound PvDBP was detected by anti-PvDBP antibodies raised in rabbits (1:5000 dilution), followed by a horseradish peroxidase–conjugated goat, anti-rabbit IgG (Millipore) at 1:5000 dilution. As a positive control we used a human monoclonal antibody against PvDBP, Sal-I variant; plasma samples from unexposed North Americans served as negative controls. Net sample absorbance values (measured at 450 nm) were obtained after subtracting background absorbance readings from wells with neither nDARC-Fc nor plasma. The percentage of binding inhibition was calculated as 1 − (net sample absorbance/net control absorbance) × 100. To assess the variant specificity of BIAbs, we tested serially diluted plasma samples (from 1:20 to 1:640) from selected study participants and nonexposed controls for their ability to inhibit the binding of 3 PvDBP recombinant proteins (variants Sal I, P, and C) to solid-phase nDARC-Fc. The BIAb end-point titer was defined as the reciprocal of the highest plasma dilution at which a blocking-inhibitory activity ≥90% could still be detected.
pvdbp Gene Sequencing
To determine whether PvDBP variants in Remansinho differed from those expressed as recombinant antigens, we sequenced the domain II (nucleotide residues 870–1545) of the pvdbp gene from 46 local P. vivax isolates collected between 2010 and 2011. The 676–base pair target sequence was amplified with PCR [20] and sequenced on an ABI Prism 3100 Genetic Analyzer (Applied Biosystems). Nucleotide sequences were deposited into the GenBank database (accession Nos. KP036999–KP037006).
Statistical Analysis
All analyses were performed with R statistical software (version 3.3.0). Proportions were compared using standard χ2 tests or Mantel-Haenszel χ2 tests for linear trend, while continuous variables were compared using the nonparametric Mann–Whitney U test. Correlations were evaluated using nonparametric Spearman correlation. Statistical significance was defined at the 5% level; 95% confidence intervals (CIs) and interquartile ranges were estimated whenever appropriate.
Survival analysis was used to compare the time to the first clinical vivax malaria episode in subjects with varying levels of specific antibodies. The outcome was clinical vivax malaria, defined as a laboratory-confirmed infection with P. vivax, regardless of parasite density, in a subject with fever, headache, or any other malaria-related symptoms or and signs at enrollment or reporting these symptoms in the past 48 hours. At each cross-sectional survey, study participants who were free of malaria infection (negative qPCR and microscopy) had their antibody levels measured. They were followed up until the next antibody status measurement. The unit of analysis was the time (in days) between the first (baseline) and the next antibody measurement, between the baseline antibody measurement and the date when subjects left the study or between the baseline antibody measurement and the next vivax malaria episode, whatever came first; each study participant contributed up to 7 observation periods.
We used mixed-effects Cox proportional hazards models to compare hazard ratios (HRs) for the time to the first clinical vivax malaria episode across terciles of antibody levels while adjusting for subjects' age and sex and for DARC genotype; the clustering of repeated observations within individuals at different time points was modeled as a random effect [21]. DARC-negative subjects were excluded from survival analysis because they are at negligible risk of vivax malaria [7]; all DARC-positive subjects (regardless of their genotype) were included. A similar approach was used to analyze HRs for the time to the first clinical vivax malaria episode according to levels of anti-PvDBP BIAbs. For this purpose, subjects were classified into terciles for the percentage of binding-inhibitory activity against the Sal I variant of PvDBP.
RESULTS
Naturally Acquired IgG Antibodies to PvDBP
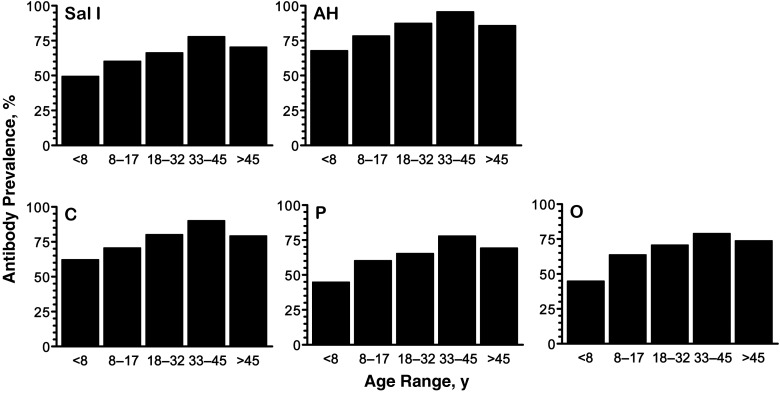
Overall, 466 study subjects were tested for antibodies at least once, 263 (56.4%) male and 203 (43.6%) female subjects with a median age of 26 years; 267 subjects (57.3%) contributed ≥2 samples. Between 62.4% and 85.6% of the 1126 plasma samples analyzed had IgG antibodies to individual PvDBP variants (Supplementary Table 1). The proportion of responders increased linearly with the age of plasma donors (P < .001 for all PvDBP variants; Figure 1) and the levels of anti-PvDBP antibodies at enrollment correlated weakly, but positively, with the subjects' age (P < .001 for all; Supplementary Table 2), consistent with a boosting effect due to repeated exposure to P. vivax.
Figure 1.
Prevalence of immunoglobulin G antibodies to 5 Plasmodium vivax Duffy binding protein (PvDBP) variants (Sal I, AH, C, P, and O) in rural Amazonians according to age. Study participants had 1–7 consecutive samples tested for antibodies; this figure shows antibody prevalence data at enrollment for 451 participants with known age. Age groups roughly correspond to quintiles, with 87–91 subjects in each group. Extended Mantel-Haenszel χ2 tests for linear trend showed a significant increase in antibody prevalence with increasing age for all PvDBP variants (P < .001).
Sequence Diversity and Antibody Recognition of PvDBP Variants
None of the 8 PvDBP variants characterized in Remansinho was identical to any PvDBP variant expressed as recombinant antigen (Table 1); pairwise differences between local variants and antigens ranged from 1 (AH vs BR7) to 8 amino acid residues (P vs BR1 and BR2). Interestingly, most study subjects recognized PvDBP variants to which they are most likely not exposed. Moreover, levels of IgG antibodies to different PvDBP recombinant proteins at study subjects' enrollment were highly correlated to each other (P < .001 for all; Supplementary Table 3), suggesting that sequence diversity in PvDBP domain II of recombinant antigens had relatively little impact on specific IgG antibody measurements.
Table 1.
Polymorphic Amino Acid Residues in PvDBP Variants Expressed as Recombinant Antigens and Those Characterized in 46 P. vivax Isolates From the Study Site (Remansinho, Brazil)
| PvDBP Variant | Amino Acid Residue (Position According to Sal I Sequence)a |
Proportion in Remansinho, % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 308 | 333 | 371 | 375 | 384 | 385 | 386 | 390 | 417 | 424 | 437 | 447 | 503 | ||
| Sal I | R | L | K | N | D | E | K | R | N | L | W | S | I | 0 |
| AH | S | … | E | … | G | … | Q | … | K | I | R | … | K | 0 |
| C | S | … | … | … | G | … | Q | … | K | I | R | … | K | 0 |
| O | S | … | … | … | G | … | … | H | … | I | … | K | … | 0 |
| P | S | F | … | D | G | K | N | H | K | I | R | … | K | 0 |
| BR1 | … | … | E | … | G | … | … | … | K | I | R | … | … | 34.8 |
| BR2 | … | … | … | … | G | … | … | H | … | … | … | … | K | 23.9 |
| BR3 | … | … | … | … | G | K | N | H | … | … | R | … | K | 15.2 |
| BR4 | S | … | E | … | G | K | N | … | K | I | R | … | K | 10.9 |
| BR5 | S | … | … | … | G | K | N | H | … | I | … | … | K | 8.7 |
| BR6 | … | … | … | … | G | … | … | … | K | I | R | … | … | 2.2 |
| BR7 | S | … | E | … | G | … | … | … | K | I | R | … | K | 2.2 |
| BR8 | … | … | … | … | G | K | … | H | … | … | R | … | … | 2.2 |
Abbreviation: PvDBP, Plasmodium vivax Duffy binding protein.
a Ellipses indicate identity with the Sal I sequence. Sal I, AH, C, O, and P are PvDBP haplotypes expressed as recombinant antigens for antibody detection in this study; BR1–BR8 are the PvDBP haplotypes found in the P. vivax population of the study site.
Antibody Responses to Other Malaria Antigens
IgG response rates were high for PvMSP-119 (67.5%) but substantially lower for PvMSP-3α and PvMSP-9 (Supplementary Table 1). Only 32.6% of the samples had IgG antibodies to PfEBA-175, a member of the Duffy binding–like erythrocyte-binding protein (erythrocyte-binding antigen) family of P. falciparum with no known orthologue with significant sequence similarity in P. vivax. Levels of IgG antibodies to PvMSP-119, PvMSP-9, and PfEBA-175, but not to PvMSP-3α, correlated weakly but positively with the subjects' age (Supplementary Table 2).
Malaria Prevalence and Antibody Levels Over Time
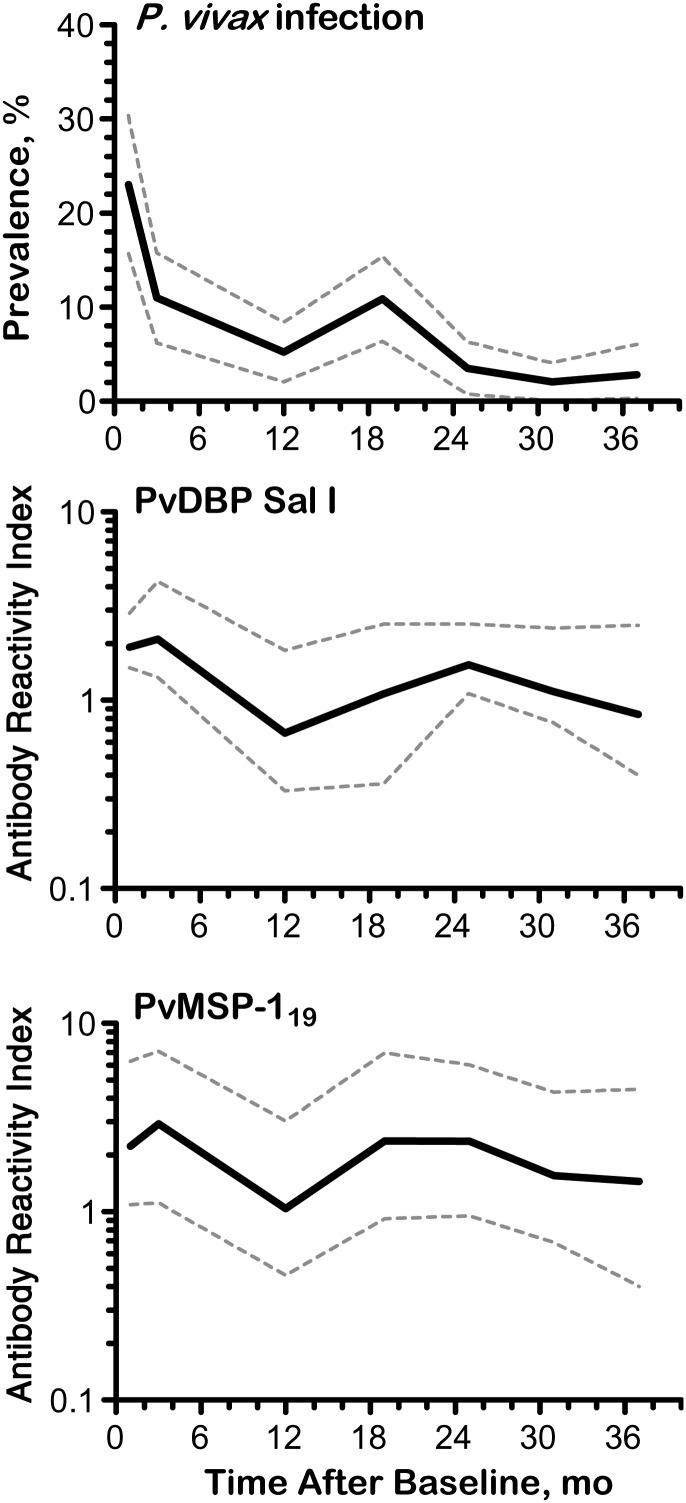
The prevalence of laboratory-confirmed P. vivax infection (positive by microscopy and/or qPCR) varied markedly in Remansinho over 37 months of study, with an outbreak due to a near-clonal parasite expansion [22] around October 2011 (Figure 2, top panel). Accordingly, levels of IgG antibodies to PvDBP and PvMSP-119 (Figure 2; see also Supplementary Figure 2) mirrored changes in P. vivax prevalence over time, declining after the baseline survey but increasing after the outbreak. Very few P. falciparum infections were diagnosed during the first year of study, and none thereafter (Supplementary Figure 2). Median IgG antibody levels to PvMSP-3α, PvMSP-9, and PfEBA-175 declined over time, with little or no increase during or after the P. vivax outbreak (Supplementary Figure 2). To further explore the antibody boosting effect of malaria episodes, we compared antibody levels in paired plasma samples collected before and after a laboratory-confirmed P. vivax infection (Supplementary Methods) and observed increased levels of antibodies to PvDBP variants and PvMSP-119 about 50 days after infection (Supplementary Figure 3). No similar increase in antibody response to PvMSP-3α, PvMSP-9 (Supplementary Figure 3) or PfEBA-175 (data not shown) was observed after a P. vivax infection.
Figure 2.
Temporal variation in Plasmodium vivax prevalence and levels of immunoglobulin (Ig) G antibodies to P. vivax Duffy binding protein (PvDBP) Sal I and the C-terminal, 19-kDa region of P. vivax merozoite surface protein 1 (PvMSP-119) in rural Amazonians. Top panel, Prevalence of P. vivax parasitemia detected with microscopy and/or quantitative polymerase chain reaction (qPCR) in 7 consecutive cross-sectional surveys in March–May 2010 (baseline), May–July 2010, March–April 2011, October–November 2011, April–May 2012, October–November 2012, and April–May 2013 (solid line); dashed lines represent 95% confidence intervals lines. Middle and bottom panels, Median reactivity indices of IgG antibodies to PvDBP Sal I (middle) and PvMSP-119 (bottom), detected with a multiplex assay during the same cross-sectional surveys (solid lines); dashed lines represent interquartile ranges. Data for other PvDBP variants and merozoite surface protein antigens are shown in Supplementary Figure 2.
Antibody Levels and Prospective Risk of Clinical Vivax Malaria
An average of 8.4 clinical vivax malaria episodes per 100 person-months at risk were recorded in the study population over the study period, with a peak of 39.3 cases per 100 person-months in October 2011 (Supplementary Figure 4). A total of 529 clinical vivax malaria episodes were recorded over the whole study period. Survival analysis showed no delay in time to the first clinical vivax malaria episode in the upper and middle terciles of IgG response to any PvDBP variant or MSP, compared with the lower tercile of antibody response (Supplementary Figure 5). We compared HRs for the time to the first clinical vivax malaria episode across terciles of specific antibody levels, using Cox proportional hazards models adjusted for age, sex, and DARC genotype, and found no significant association between levels of specific IgG antibodies and prospective risk of clinical vivax malaria (Table 2).
Table 2.
Association Between Levels of Naturally Acquired Antibodies to PvDBP and PvMSP Variants and Prospective Risk of Clinical Vivax Malaria in Rural Amazonians
| Antigen | Variant | Middle vs Lower Tercile of Reactivity Indicesa |
Upper vs Lower Tercile of Reactivity Indicesa |
||
|---|---|---|---|---|---|
| HR (95% CI)b | P Value | HR (95% CI)b | P Value | ||
| PvDBP | Sal I | 1.05 (.73–1.51) | .78 | 1.14 (.78–1.67) | .49 |
| AH | 0.88 (.62–1.25) | .47 | 0.88 (.60–1.27) | .49 | |
| C | 0.91 (.64–1.29) | .60 | 0.97 (.67–1.40) | .89 | |
| O | 0.83 (.59–1.16) | .28 | 0.89 (.61–1.26) | .48 | |
| P | 0.81 (.56–1.15) | .25 | 0.93 (.64–1.34) | .69 | |
| PvMSP-119 | Belém | 0.82 (.58–1.16) | .26 | 1.20 (.85–1.68) | .31 |
| PvMSP-3α | Belém | 1.11 (.76–1.61) | .60 | 1.21 (.83–1.77) | .33 |
| PvMSP-9 | Belém | 0.86 (.60–1.24) | .42 | 1.01 (.72–1.43) | .93 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PvDBP, Plasmodium vivax Duffy binding protein; P. vivax PvMSP, merozoite surface protein.
a Antibody levels were stratified into terciles of reactivity indices for analysis.
b HRs were obtained with Cox proportional hazards models adjusted for age, sex, and genotype for the Duffy antigen/receptor for chemokines.
Naturally Acquired Anti-PvDBP BIAbs
We used the Sal I variant of PvDBP to measure BIAbs in 572 plasma samples from 263 subjects (1–7 samples per subject; 47.9% of subjects had ≥2 samples tested) with detectable antibody responses to PvDBP using the bead array assay. The frequency distribution of BIAb levels at subjects' enrollment was bimodal, with peaks around 0%–10% and 90%–100% inhibition (Figure 3A); 26.6% of the samples had >80% and 20.5% had >90% BIAb activity. BIAb levels correlated positively with levels of IgG antibodies to all PvDBP variants (P < .001 for all; Supplementary Table 4). Furthermore, BIAb activity correlated weakly, but positively, with subjects' age (ρ = 0.247; P = .001). To examine whether high levels of binding-inhibitory activity changed over time, we chose 43 study participants with ≥1 BIAb activity measurement above 80% and analyzed their subsequent BIAb levels. Thirty subjects (69.8%) maintained their inhibitory activity at >80% in all subsequent evaluations up to 37 months apart (total of 2–6 measurements per subject), and only 13 subjects had BIAb activity reduced to ≤80% during the follow-up (Supplementary Figure 6). We found no significant boosting of BIAb responses after a laboratory-confirmed P. vivax infection documented during follow-up (data not shown). These results indicate that, once acquired, high levels of binding-inhibitory activity usually persist for months, even in the absence of repeated exposure to the parasite.
Figure 3.
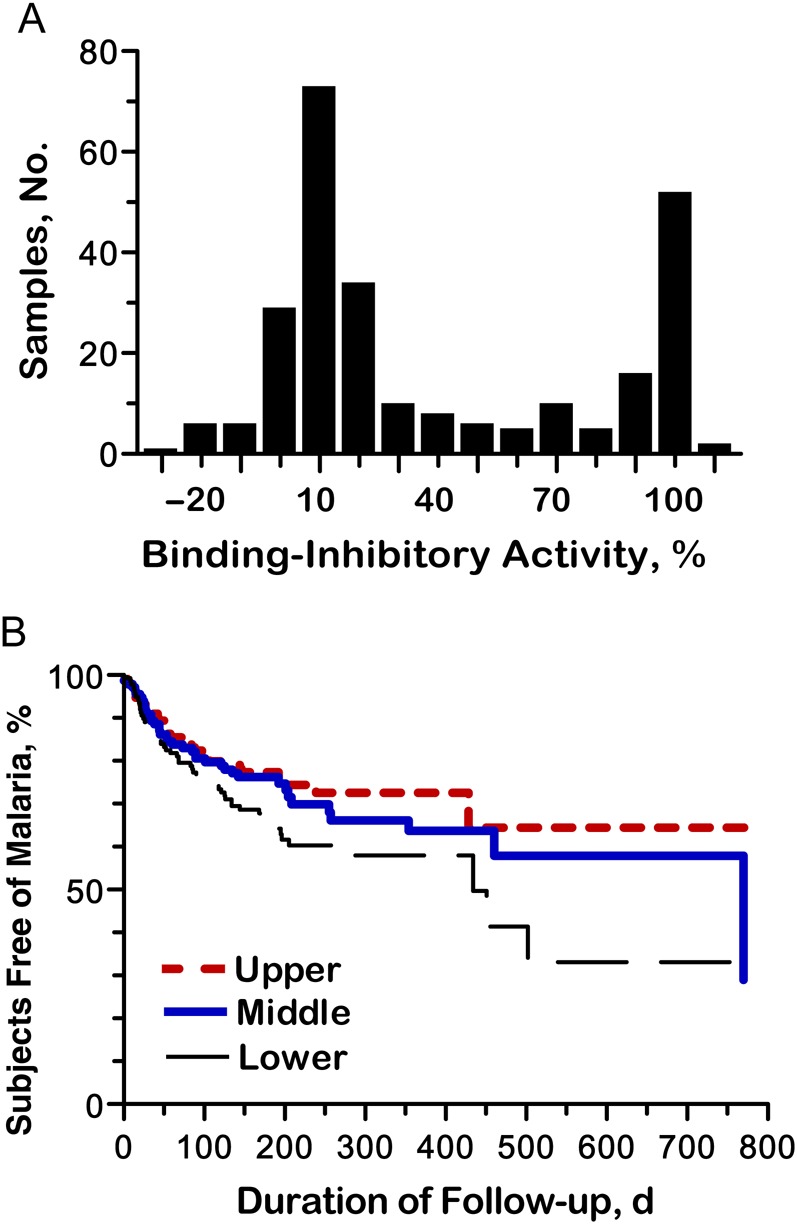
Naturally acquired binding-inhibitory antibodies (BIAbs) to Plasmodium vivax Duffy binding protein (PvDBP) in rural Amazonians. BIAb activity was expressed as the percentage of inhibition of PvDBP (Sal I variant) binding to solid-phase Duffy antigen/receptor for chemokines (DARC) in vitro. A, Frequency distribution of the percentage of binding inhibition of anti-PvDBP antibodies at enrollment in 263 rural Amazonians; quite similar results were obtained with the complete data set (n = 572 samples). B, Kaplan–Meier curve showing the proportion of rural Amazonians who remained free of microscopically confirmed clinical vivax malaria during follow-up, according to their levels of anti-PvDBP BIAbs. Study participants (n = 229) who were DARC positive and free of P. vivax infection at the time of antibody measurement were grouped into terciles of BIAb activity (upper, ≥50%; middle, 6%–49%; lower, <6%) and followed up for up to 37 months. The unit of analysis was the period of observation (in days) between the baseline and the next BIAb measurement, the first vivax malaria episode, or the date when subjects left the study, whichever came first. Each subject contributed between 1 and 6 periods of observation, with 456 BIAb measurements (n = 152 in each BIAb tercile) and 126 events (33, 39, and 54 in the upper, middle, and lower BIAb terciles, respectively) analyzed.
Variant Specificity of BIAbs
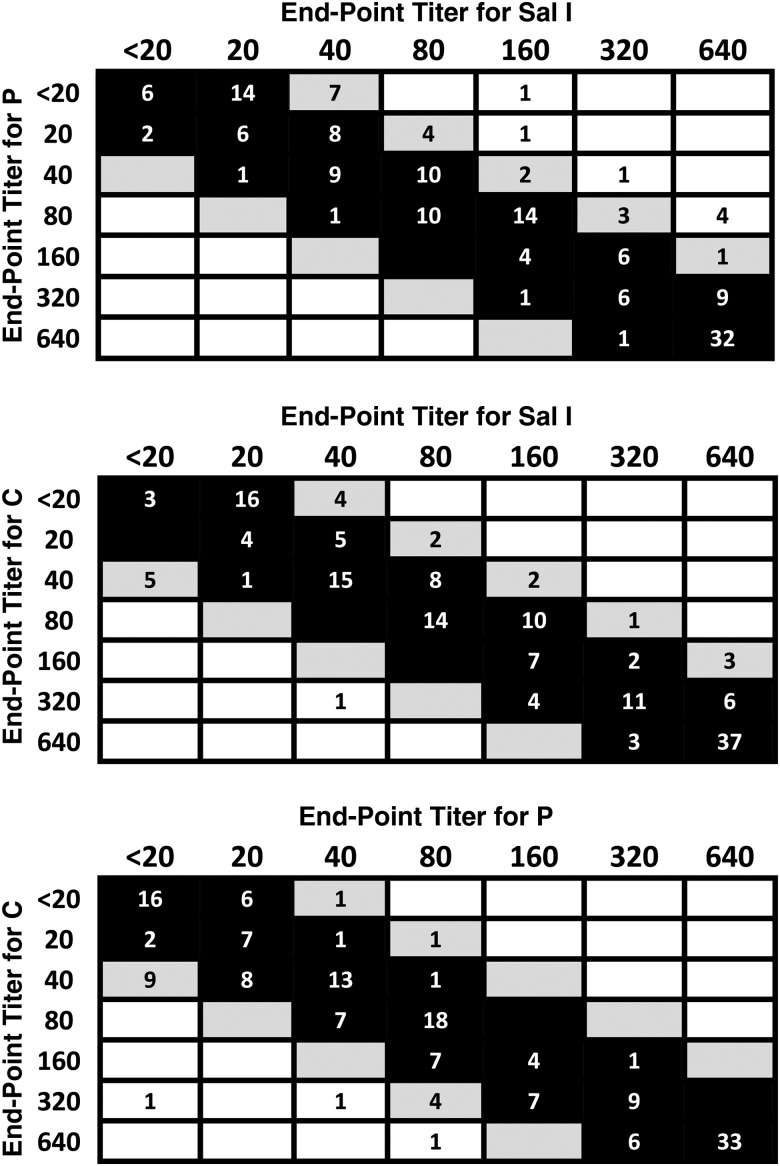
We used 164 plasma samples from 77 subjects with BIAb activity of >80%, measured with the Sal I variant of PvDBP, to evaluate the variant specificity of BIAbs. Plasma samples were serially diluted between 1:20 and 1:640 to compare their binding-inhibitory end-point titers with PvDBP variants Sal I, C, and P, using the same functional assay. The vast majority (82.9%–89.0%) of plasma samples had similar binding-inhibitory activity against each variant (≤2-fold difference in pairwise comparisons of end-point titers; Figure 4), despite the substantial divergence in their domain II sequences (Table 1). Some (9.1%–16.5%) had moderately variant-specific BIAbs, with a 4-fold difference in end-point titer to pairs of variants, while few samples (0.6%–4.3%) had highly variant-specific BIAbs with >4-fold difference in end-point titer (Figure 4). These data indicate that, once acquired, high levels of binding-inhibitory activity for anti-PvDBP antibodies are mostly variant transcending.
Figure 4.
Pairwise comparisons of binding-inhibitory antibody (BIAb) end-point titers to Plasmodium vivax Duffy binding protein variants in rural Amazonians. Top panel, Sal I variant (columns) versus P variant (rows). Middle panel, Sal I variant (columns) versus C variant (rows). Bottom panel, P variant (columns) versus C variant (rows). Black boxes indicate comparisons with a ≤2-fold difference in end-point titer between variants (“variant-transcending” BIAbs); gray boxes, comparisons with a 4-fold difference in end-point titer (“moderately variant-specific” BIAbs); and white boxes, comparisons with a >4-fold difference in end-point titer (“variant-specific” BIAbs). Numbers of samples with each end-point titer are indicated within boxes; results for 164 plasma samples from 77 subjects (1–7 samples per study participant) are analyzed.
BIAbs and Prospective Risk of Clinical Vivax Malaria
We next tested whether strong BIAb responses protected from clinical malaria. To this end, we grouped study participants into terciles of BIAb response. Survival analysis showed a delay in time to the first clinical vivax malaria episode among subjects in the upper and middle terciles of BIAb response, compared with their counterparts in the lower tercile (Figure 3B). A Cox proportional hazards model adjusted for age, sex, and DARC genotype showed a 41%–42% reduction in the prospective risk of clinical malaria among subjects in the upper and middle terciles of BIAb response at baseline, relative to those in the lower tercile (Table 3). Age (in years) was another independent predictor of reduced risk of clinical vivax malaria in the fully adjusted Cox model (HR, 0.98; 95% CI, .97–1.00; P = .005), suggesting that age-related factors other than BIAbs are also associated with protection. Female sex (HR, 1.45; 95% CI, 1.01–2.08; P = .04) was independently associated with an increased risk of malaria, after adjustment for BIAb response, but we have no clear-cut explanation for this finding. Interestingly, the Cox model revealed no significant interaction between DARC genotype and BIAb response (P values between .27 and .98) in their association with the outcome, despite the recent finding of greater in vitro binding-inhibitory activity of anti-PvDBP antibodies with Fya RBCs, compared with Fyb RBCs [23].
Table 3.
Association Between Binding-Inhibitory Activity of Naturally Acquired Antibodies to PvDBP and Prospective Risk of Clinical Vivax Malaria in Rural Amazonians
| Cox Model | Middle vs Lower Tercile of BIAb Responsesa |
Upper vs Lower Tercile of BIAb Responsesa |
||
|---|---|---|---|---|
| HR (95% CI)b | P Value | HR (95% CI)b | P Value | |
| Unadjusted | 0.59 (.39–.90) | .01 | 0.51 (.33–.79) | .003 |
| Adjusted for age | 0.62 (.41–.94) | .02 | 0.59 (.38–.93) | .02 |
| Adjusted for sex | 0.57 (.43–.97) | .04 | 0.48 (.37–.86) | .008 |
| Adjusted for DARC genotype | 0.56 (.37–.86) | .008 | 0.54 (.34–.84) | .007 |
| Adjusted for all variables above | 0.58 (.38–.89) | .01 | 0.59 (.37–.93) | .02 |
Abbreviations: BIAb, binding-inhibitory antibody; CI, confidence interval; DARC, Duffy antigen/receptor for chemokines; HR, hazard ratio; PvDBP, Plasmodium vivax Duffy binding protein.
a Antibody responses were stratified into terciles of the percentage of binding inhibition: upper (≥50%), middle (6%–49%), and lower (<6%).
b HRs were obtained with Cox proportional hazards models.
DISCUSSION
This cohort study provides the first evidence that naturally acquired anti-PvDBP BIAb responses are associated with reduced risk of clinical vivax malaria. Of note, high BIAb responses developed under conditions of low malaria endemicity that are typical of rural Amazonian communities and, once acquired, were predominantly variant transcending and long lasting. Moreover, the protective effect of anti-PvDBP BIAbs was not modulated by hosts' DARC genotype. These results further support PvDBP as the most promising vaccine target for P. vivax blood stages.
Only 8.7% of children aged 5–14 years and exposed to very intense malaria transmission in Papua New Guinea have been found to acquire BIAbs with >90% inhibitory activity [9]. Interestingly, these children with high levels of BIAbs at baseline had a reduced risk of P. vivax malaria diagnosed by means of conventional microscopy over the next 25 weeks, compared with those with <50% inhibitory activity, although no protection was observed against P. vivax infections diagnosed with a more sensitive molecular method. Moreover, these children, once infected, had comparatively reduced P. vivax densities [9]. These results indicate that high levels of BIAbs in hyperendemic settings are associated with reduced parasite growth, often resulting in low-density P. vivax infections that are missed by conventional microscopy, rather than fully sterilizing immunity. This previous study was unable to examine the association of high levels of BIAbs with protection against clinical immunity because by age 5 years almost all the children in this population were immune to P. vivax illness.
Our data underline the need for functional assays to detect anti-PvDBP antibodies mediating protective immunity, rather than antibodies that are markers of increased exposure to infection [24]. We found no association between high total IgG responses to PvDBP and clinical immunity to malaria in rural Amazonians, suggesting that our conventional serology measures both protective and nonprotective antibodies. Whether BIAbs target mostly polymorphic or conserved epitopes of PvDBP domain II remains to be determined, but our titration experiments with different PvDBP variants suggest that most strongly inhibitory antibodies are variant transcending. To better analyze the fine specificity of naturally acquired BIAbs, we are currently generating a panel of human monoclonal antibodies, from rural Amazonians whose anti-PvDBP antibodies have high and long-lasting binding-inhibitory activity, for use in epitope mapping and further functional characterization of these potentially protective responses.
Given our inability to distinguish protective from nonprotective antibodies by conventional serology, the absence of significant associations between IgG responses to PvMSP-119, PvMSP-3α, and PvMSP-9 and protection from clinical vivax malaria is not entirely surprising. A single cohort study of young Papua New Guinean children aged 1–3 years has shown an association between high levels of antibodies to both PvMSP-3α and PvMSP-9 and reduced incidence of clinical vivax malaria over 16 months of follow-up [25]. Of note, no association between antibody responses to PvMSP-119 and reduced risk of P. vivax infection [8, 26, 27] or high-density parasitemia [8, 27] has been found in cohort studies so far. In contrast, the association between anti-PfMSP-119 antibodies and protection from P. falciparum malaria has been well demonstrated in Africa and Papua New Guinea [28].
We conclude that high titers of long-lasting BIAbs develop and can confer broadly specific clinical immunity to P. vivax under conditions of low malaria transmission, with clear implications for the development of PvDBP-based vaccines. As shown elsewhere [29], repeated exposure to the parasite is not necessarily required to maintain strong BIAb responses, once they develop. Moreover, BIAbs seem to protect against several PvDBP variants, putatively because they target conserved, rather than variant-specific epitopes [24]. Therefore, a PvDBP vaccine that elicits antibody responses with these properties is very likely to be successful in preventing P. vivax infection and clinical disease.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all inhabitants in Remansinho for their enthusiastic participation in this study; Amanda B. Gozze, Pablo S. Fontoura, Kézia Katiani G. Scopel, Nathália F. Lima, Carlos E. Cavasini, Raquel M. Gonçalves, Maria José Menezes, Rosely S. Malafronte, Camilla L. Batista, Ariel M. Silber, Cristiana F. Alves de Brito, Mônica da Silva-Nunes, Carla Roberta O. Carvalho, and Mauro R. Tucci for the help in field work and clinical care of patients; Cleide F. Nunes and Eusueli Arraes da Silva for microscopic diagnosis of malaria; Márcio C. Santana, Andrecresa N. Duarte, and Francisco Naildo C. Leitão for overall logistic support; and Lenore Carias for laboratory support.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (International Centers of Excellence in Malaria Research program; research grants U19 AI089681 to Joseph M. Vinetz, U19 AI089686 to James W. Kazura, and AI064478 to C. L. K.); the Veterans Administration Research Service (C. L. K.), the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (grants 2009/52729-9 to M. U. F. and 2013/23770-6 to S. B.), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico of Brazil (scholarship to V. C. N. and senior researcher scholarship to M. U. F.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Longley RJ, Sattabongkot J, Mueller I. 2016. Insights into the naturally acquired immune response to Plasmodium vivax malaria. Parasitology 2016; 143:154–70. [DOI] [PubMed] [Google Scholar]
- 2.Lin E, Kiniboro B, Gray L et al. . Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One 2010; 5:e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg 2002; 66:641–8. [DOI] [PubMed] [Google Scholar]
- 4.da Silva-Nunes M, Codeço CT, Malafronte RS et al. . Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg 2008; 79:624–35. [PubMed] [Google Scholar]
- 5.Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon basin of Brazil. Am J Trop Med Hyg 2009; 80:452–9. [PubMed] [Google Scholar]
- 6.Horuk R, Chitnis CE, Darbonne WC et al. . A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 1993; 261:1182–4. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman PA, Ferreira MU, Howes RE, Mercereau-Puijalon O. Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv Parasitol 2013; 81:27–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole-Tobian JL, Michon P, Biasor M et al. . Strain-specific Duffy binding protein antibodies correlate with protection against infection with homologous compared to heterologous Plasmodium vivax strains in Papua New Guinean children. Infect Immun 2009; 77:4009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King CL, Michon P, Shakri AR et al. . Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci U S A 2008; 105:8363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimberg BT, Udomsangpetch R, Xainli J et al. . Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med 2007; 4:e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa S, Gozze AB, Lima NF et al. . Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis 2014; 8:e3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonçalves RM, Scopel KK, Bastos MS, Ferreira MU. Cytokine balance in human malaria: does Plasmodium vivax elicit more inflammatory responses than Plasmodium falciparum? PLoS One 2012; 7:e44394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health of Brazil. Practical guidelines for malaria therapy [in Portuguese]. Ministry of Health of Brazil, 2010. http://bvsms.saude.gov.br/bvs/publicacoes/guia_pratico_malaria.pdf. Accessed 30 May 2016. [Google Scholar]
- 14.Kempińska-Podhorodecka A, Knap O, Drozd A et al. . Analysis for genotyping Duffy blood group in inhabitants of Sudan, the fourth cataract of the Nile. Malar J 2012; 11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Pandey K, Chattopadhayay R et al. . Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax Duffy-binding protein. J Biol Chem 2001; 276:17111–6. [DOI] [PubMed] [Google Scholar]
- 16.Lima-Junior JC, Jiang J, Rodrigues-da-Silva RN et al. . B cell epitope mapping and characterization of naturally acquired antibodies to the Plasmodium vivax merozoite surface protein-3α (PvMSP-3α) in malaria exposed individuals from Brazilian Amazon. Vaccine 2011; 29:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima-Junior JC, Tran TM, Meyer EV et al. . Naturally acquired humoral and cellular immune responses to Plasmodium vivax merozoite surface protein 9 in Northwestern Amazon individuals. Vaccine 2008; 26:6645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dent AE, Malhotra I, Wang X et al. . Contrasting patterns of serologic and functional antibody dynamics to Plasmodium falciparum antigens in a Kenyan birth cohort. Clin Vaccine Immunol 2015; 23:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakri AR, Rizvi MM, Chitnis CE. Development of quantitative receptor-ligand binding assay for use as a tool to estimate immune responses against Plasmodium vivax Duffy binding protein region II. J Immunoassay Immunochem 2012; 33:403–13. [DOI] [PubMed] [Google Scholar]
- 20.Sousa TN, Cerávolo IP, Fernandes Fontes CJ, Couto A, Carvalho LH, Brito CF. The pattern of major polymorphisms in the Duffy binding protein ligand domain among Plasmodium vivax isolates from the Brazilian Amazon area. Mol Biochem Parasitol 2006; 146:251–4. [DOI] [PubMed] [Google Scholar]
- 21.Vaida F, Xu R. Proportional hazards model with random effects. Stat Med 2000; 19:3309–24. [DOI] [PubMed] [Google Scholar]
- 22.Batista CL, Barbosa S, da-Silva Bastos M, Viana SA, Ferreira MU. Genetic diversity of Plasmodium vivax over time and space: a community-based study in rural Amazonia. Parasitology 2015; 142:374–84. [DOI] [PubMed] [Google Scholar]
- 23.King CL, Adams JH, Xianli J et al. . Fya/Fyb antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci U S A 2011; 108:20113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ntumngia FB, King CL, Adams JH. Finding the sweet spots of inhibition: understanding the targets of a functional antibody against Plasmodium vivax Duffy binding protein. Int J Parasitol 2012; 42:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanisic DI, Javati S, Kiniboro B et al. . Naturally acquired immune responses to P. vivax merozoite surface protein 3α and merozoite surface protein 9 are associated with reduced risk of P. vivax malaria in young Papua New Guinean children. PLoS Negl Trop Dis 2013; 7:e2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueira PA, Alves FP, Fernandez-Becerra C et al. . A reduced risk of infection with Plasmodium vivax and clinical protection against malaria are associated with antibodies against the N terminus but not the C terminus of merozoite surface protein 1. Infect Immun 2006; 74:2726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutts JC, Powell R, Agius PA, Beeson JG, Simpson JA, Fowkes FJ. Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis. BMC Med 2014; 12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med 2010; 7:e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souza-Silva FA, da Silva-Nunes M, Sanchez BA et al. . Naturally acquired antibodies to Plasmodium vivax Duffy binding protein (DBP) in Brazilian Amazon. Am J Trop Med Hyg 2010; 82:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.