Fig. 1.
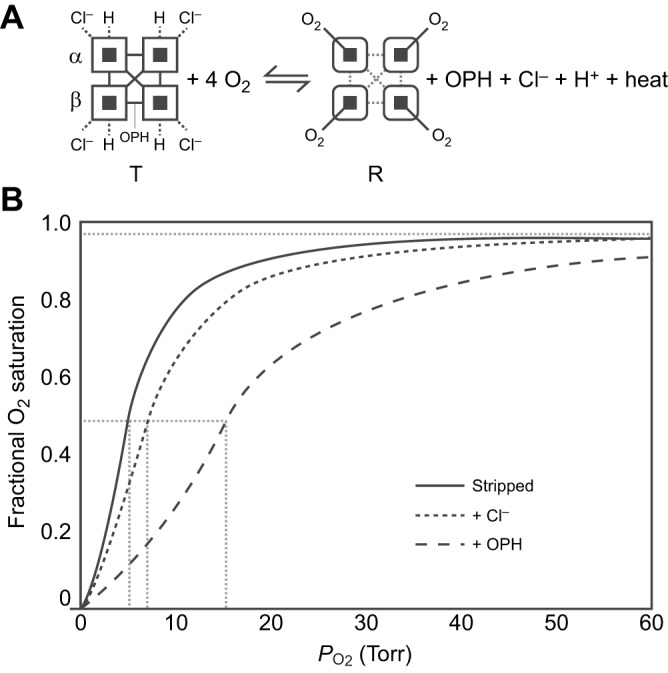
The allosteric regulation of hemoglobin (Hb)–O2 affinity. (A) The oxygenation reaction of tetrameric Hb (α2β2) involves an allosteric transition in quaternary structure from the low-affinity T-state to the high-affinity R-state. The oxygenation-induced T→R transition entails a breakage of salt bridges and hydrogen bonds within and between subunits (open squares), dissociation of allosterically bound organic phosphates (OPHs), Cl− ions and protons, and the release of heat (heme oxygenation is an exothermic reaction). Oxygenation-linked proton binding occurs at multiple residues in the α- and β-chains, Cl− binding mainly occurs at the N-terminal α-amino groups of the α- and β-chains in addition to other residues in both chains, and phosphate binding occurs between the β-chains in the central cavity of the Hb tetramer. (B) O2 equilibrium curves for purified Hb in the absence of allosteric effectors (Stripped) and in the presence of chloride ions (+Cl−) and organic phosphates (+OPH). The preferential binding of allosteric effectors to deoxyHb stabilizes the T-state, thereby shifting the allosteric equilibrium in favour of the low-affinity quaternary structure. The O2 equilibrium curves are therefore right-shifted (Hb–O2 affinity is reduced) in the presence of such effectors. Hb–O2 affinity is indexed by the P50 value (dashed grey lines) – the PO2 at which Hb is half-saturated. The sigmoidal shape of the O2 equilibrium curves reflects cooperative O2 binding, involving a PO2-dependent shift from low- to high-affinity conformations.
