Table 2.
Synthesis of pyridine‐based PSBH scaffolds.
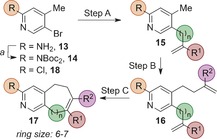
| Step | |||||||
|---|---|---|---|---|---|---|---|
| R | n | R1 | R2 | A (15)[b] | B (16)[e] | C (17)[f] | |
| a | Boc2N | 0 | H | H | 85 % | 60 % | 95 % |
| b | Boc2N | 0 | H | Me | – | 69 % | 94 % |
| c | Boc2N | 0 | H | CF3 | – | 77 % | 54 % |
| d | Boc2N | 0 | Me | H | 87 % | 60 % | 94 % |
| e | Boc2N | 0 | Ph | H | 92 % | 68 % | 83 % |
| f | Boc2N | 1 | H | H | 78 %[c] | 41 % | 65 % |
| g | Cl | 1 | H | H | 73 %[d] | 79 % | 91 % |
Reaction conditions: [a] Boc2O (2.5 equiv), DMAP (0.1 equiv), THF, 70 °C. [b] R′‐BF3K or R′‐B(MIDA) (1.5 equiv), Pd(dppf)Cl2⋅CH2Cl2 (10 mol %), K2CO3 (3.0 equiv), THF/H2O, 70 °C. [c] Allyltributyltin (1.1 equiv), Pd(PPh3)4 (10 mol %), KF (2 equiv), toluene, 110 °C. [d] i‐PrMgCl.LiCl (1.5 equiv), allyl bromide (1.2 equiv), THF, −15 °C to RT. [e] LDA (1.2 equiv), alkyl bromide (1.5 equiv), THF, −78 °C to RT. [f] Grubbs II (5 mol %), CH2Cl2, 40 °C. DMAP=4‐dimethylaminopyridine.
