Figure 1.
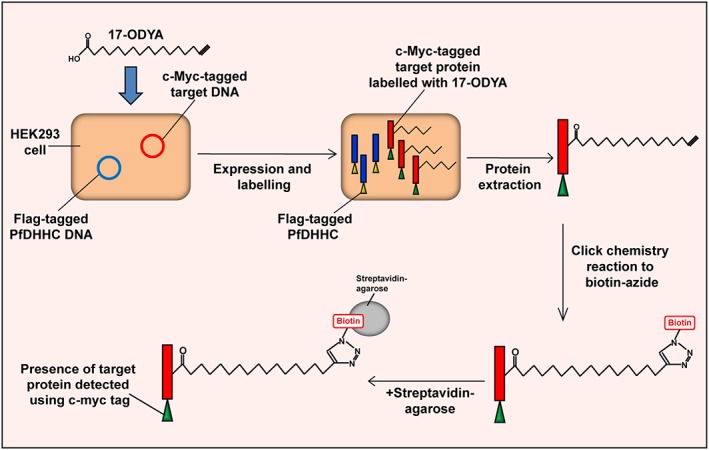
Palmitoyl‐transferase activity assay. This assay incorporates the expression of Plasmodium falciparum proteins in a mammalian cell expression system along with metabolic labelling and click chemistry methods of palmitoyl‐protein purification. Human embryonic kidney 293E (HEK293E) cells were co‐transfected with FLAG‐tagged PfDHHC DNA along with the c‐Myc‐tagged DNA of a potential target palmitoyl protein, both of which were codon‐optimized for expression in human cells. The HEK293E cells were then treated with the metabolic label, 17‐octadecynoic acid (17‐ODYA). Proteins were extracted and reacted to biotin‐azide under standard click chemistry conditions, resulting in the biotinylation of all 17‐ODYA‐labelled proteins. Biotinylated proteins were affinity purified using streptavidin‐agarose and the presence of the target protein detected using antibodies against the c‐Myc tag. As a standard control, transfected cells were also mock labelled with DMSO instead of 17‐ODYA. As a control for the background palmitoylation of target proteins by endogenous HEK293E DHHC proteins, cells were also co‐transfected with the c‐Myc‐tagged target protein and the CD4‐expressing control vector, in the absence of the PfDHHC. The level of target protein palmitoylation when in the presence of the PfDHHC compared with when in the absence of the PfDHHC was expected to be an indication of whether the particular PfDHHC was responsible for the palmitoylation of the target protein.
