Abstract
Prion diseases result from the misfolding of the normal, cellular prion protein (PrPC) to an abnormal protease resistant isomer called PrPRes. The emergence of prion diseases in wildlife populations and their increasing threat to human health has led to increased efforts to find a treatment for these diseases. Recent studies have found numerous anti-prion compounds that can either inhibit the infectious PrPRes isomer or down regulate the normal cellular prion protein. However, most of these compounds do not cross the blood brain barrier to effectively inhibit PrPRes formation in brain tissue, do not specifically target neuronal PrPC, and are often too toxic to use in animal or human subjects.
We investigated whether siRNA delivered intravascularly and targeted towards neuronal PrPC is a safer and more effective anti-prion compound. This report outlines a protocol to produce two siRNA liposomal delivery vehicles, and to package and deliver PrP siRNA to neuronal cells. The two liposomal delivery vehicles are 1) complexed-siRNA liposome formulation using cationic liposomes (LSPCs), and 2) encapsulated-siRNA liposome formulation using cationic or anionic liposomes (PALETS). For the LSPCs, negatively charged siRNA is electrostatically bound to the cationic liposome. A positively charged peptide (RVG-9r [rabies virus glycoprotein]) is added to the complex, which specifically targets the liposome-siRNA-peptide complexes (LSPCs) across the blood brain barrier (BBB) to acetylcholine expressing neurons in the central nervous system (CNS). For the PALETS (peptide addressed liposome encapsulated therapeutic siRNA), the cationic and anionic lipids were rehydrated by the PrP siRNA. This procedure results in encapsulation of the siRNA within the cationic or anionic liposomes. Again, the RVG-9r neuropeptide was bound to the liposomes to target the siRNA/liposome complexes to the CNS. Using these formulations, we have successfully delivered PrP siRNA to AchR-expressing neurons, and decreased the PrPC expression of neurons in the CNS.
Keywords: Bioengineering, Issue 113, neurodegeneration, therapeutics, prion, siRNA, liposomes, protamine sulfate, blood brain barrier, brain
Introduction
Prions are severe neurodegenerative diseases that affect the CNS. Prion diseases result from the misfolding of the normal cellular prion protein, PrPC, by an infectious isomer called PrPRes. These diseases affect a wide variety of species including bovine spongiform encephalopathy in cows, scrapie in sheep, chronic wasting disease in cervids, and Creutzfeldt-Jakob disease in humans1-3. Prions cause neurodegeneration that starts with synaptic loss, and progresses to vacuolization, gliosis, neuronal loss, and plaque deposits. Eventually, resulting in the death of the animal/individual4. For decades, researchers have investigated compounds meant to slow or stop the progression of prion disease. However, researchers have not found either a successful therapy or an effective systemic delivery vehicle.
Endogenous PrPC expression is required for the development of prion diseases5. Therefore, decreasing or eliminating PrPC expression may result in a delay or amelioration of disease. Several groups created transgenic mice with reduced levels of PrPC or injected lentivectors expressing shRNA directly into murine brain tissue to investigate the role of PrPC expression levels in prion disease. These researchers found reducing the amount of neuronal PrPC resulted in halting the progressive neuropathology of prion diseases and extended the life of the animals6-9. We have reported that PrPC siRNA treatment results in clearance of PrPRes in mouse neuroblastoma cells10. These studies suggest that the use of therapies to decrease PrPC expression levels, like small interfering RNA (siRNA), which cleaves mRNA, may sufficiently delay the progression of prion diseases. However, most therapies investigated for prion diseases were delivered in ways that would not be practical in a clinical setting. Therefore, a siRNA therapy needs a systemic delivery system, which is delivered intravenously and targeted to the CNS.
Investigators have studied the use of liposomes as delivery vehicles for gene therapy products. Cationic and anionic lipids are both used in the formation of liposomes. Cationic lipids are more widely used than anionic lipids because the charge difference between the cationic lipid and the DNA/RNA allows for efficient packaging. Another advantage of cationic lipids is that they cross the cell membrane more easily than other lipids11-14. However, cationic lipids are more immunogenic than anionic lipids13,14. Therefore, researchers have started to shift from using cationic to anionic lipids in liposomes. Gene therapy products can be efficiently packaged into anionic liposomes using the positively charged peptide protamine sulfate, which condenses DNA/RNA molecules15-19. Since anionic lipids are less immunogenic than cationic lipids they may have increased circulation times, and may be more tolerated in animal models13,14. Liposomes are targeted to specific tissues using targeting peptides that are attached to the liposomes. The RVG-9r neuropeptide, which binds to nicotinic acetylcholine receptors, has been used to target siRNA and liposomes to the CNS17-20.
This report outlines a protocol to produce three siRNA delivery vehicles, and to package and deliver the siRNA to neuronal cells (Figure 1). Liposome-siRNA-peptide complexes (LSPCs) are composed of liposomes with siRNA and the RVG-9r targeting peptide electrostatically attached to the outer surface of the liposome. Peptide addressed liposome encapsulated therapeutic siRNA (PALETS) are composed of siRNA and protamine encapsulated within the liposome, with RVG-9r covalently bonded to lipid PEG groups. Using the below methods to generate LSPCs and PALETS, PrPC siRNA decreases PrPC expression up to 90% in neuronal cells, which holds tremendous promise to cure or substantially delay the onset of prion disease pathology.
Protocol
All mice were bred and maintained at Lab Animal Resources, accredited by the Association for Assessment and Accreditation of Lab Animal Care International, in accordance with protocols approved by the Institutional Animal Care and Use Committee at Colorado State University.
1. Preparation of LSPCs
Use a 1:1 DOTAP (1,2-dioleoyl-3-trimethylammonium-propane):cholesterol ratio for LSPCs. For a 4 nmole liposome preparation, mix 2 nmoles of DOTAP and 2 nmoles of cholesterol into 10 ml of a 1:1 chloroform:methanol solution in a flask.
Evaporate 9 ml of the chloroform:methanol solution using N2 gas in a fume hood. Evaporate the last 1 ml of solvent in a fume hood overnight without gas. Observe a thin lipid film on the bottom of the flask.
Heat 10 ml of a 10% sucrose solution to 55 °C.
Pour the heated 10% sucrose solution onto the thin lipid film slowly, i.e. 1 ml/min, while gently swirling the flask. Maintain the 10% sucrose and flask with lipids at 55 °C so that the lipid molecules remain in the gel-liquid phase. Rehydration results in multilamellar vesicle (MLV) formation.
Allow lipids to rehydrate at 55 °C for at least one hr before sizing the liposomes.
Assemble an extruder per manufacturer's instructions with 1.0 µm filters or use 1.0 µm syringe filters for sizing.
Add 1 ml of the heated liposome suspension to one of the syringes on the extruder, and pass the suspension between the two syringes 11 times. Make sure that the suspension is in the opposite syringe than the starting syringe after the 11 passes.
Size the liposomes again through 0.45 µm then 0.2 µm filters to generate large unilamellar vesicles (LUVs). Keep the liposome suspension heated for easier sizing.
Incubate 20 µl of the 4 nmole liposome suspension (200 µM) with 200 µl of a 20 µM (4 nmole total) stock siRNA solution for 10 min at room temperature.
Incubate 80 µl of a 500 µM (40 nmole total) stock RVG-9r solution with the siRNA/liposome solution for 10 min at RT. LSPCs should be used as soon as possible but can be used up to several hours after preparation. Store LSPCs at 4 °C for several hr after preparation.
2. Preparation of PALETS
Use a 55:40:5 molar ratio of either DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine):cholesterol:DSPE-PEG or DOTAP:cholesterol:DSPE-PEG for PALETS. For a 4 nmole liposome preparation, mix 2.2 nmoles of either DSPE or DOTAP, 1.6 nmoles of cholesterol, and 0.2 nmoles of DSPE-PEG into 10 ml of a 2:1 chloroform:methanol solution in a flask.
Prepare liposome suspension exactly as described in Step 1.1 and 1.2 above. Rehydrate DSPE liposomes in 10 ml of 1x PBS heated to 75 °C, adding 1 ml/min. Allow lipids to rehydrate at 75 °C for at least 1 hr before sizing.
Size the liposome suspension exactly as described in Step 1.6-1.8.
Pipet 20 µl of each of the 4 nmole (200 µM) liposome suspensions into single use aliquots after DOTAP and DSPE liposomes have been sized, and lyophilize for 30 min using a benchtop freeze dryer (Figure 2).
Incubate 200 µl of a 20 µM (4 nmole total) stock siRNA solution with 1.4 µl of a 26.6 µM solution of protamine sulfate for 10 min at RT for siRNA that is to be encapsulated into DSPE PALETS liposomes.
Rehydrate the DOTAP PALETS liposomes with 200 µl of a 4 nmole stock solution of siRNA or rehydrate DSPE PALETS liposomes with 201.4 µl of the siRNA/protamine solution. Incubate liposome/siRNA solution for 10 min at RT.
Add 10 µl of a 60 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) solution to the liposome/siRNA solution for both DOTAP and DSPE PALETS.
Add 10 µl of a 150 mM N-hydroxysulfosuccinimide (sulfo-NHS) solution to the liposome/siRNA solution for both DOTAP and DSPE PALETS.
Incubate the EDC and sulfo-NHS with the siRNA/liposome suspension for 2 hr at RT.
Incubate 80 µl of a 500 µM (40 nmole total) stock RVG-9r solution with the siRNA/liposome crosslinking solution for 10 min at RT. NOTE: The EDC/sulfo-NHS allows the RVG-9r to covalently bond to the PEG lipids. Use PALETS within several hr after preparation. Store PALETS at 4 °C for several hr after preparation.
- To determine siRNA encapsulation efficiency of PALETS:
- Measure the concentration of siRNA before and after the addition of protamine and liposomes using a spectrophotometer set at 260 nm.
- Filter the unencapsulated siRNA from the liposome/encapsulated siRNA suspension using 50 kDa centrifugal filters. Centrifuge at 1,000 x g for 20 min.
- Measure the concentration of unencapsulated siRNA in the filtrate and the concentration of encapsulated siRNA in the retentate using a spectrophotometer at 260 nm.
3. Injecting Mice with LSPCs or PALETS
Expose mice for 5-10 min to a heat lamp to dilate vasculature.
Anesthetize mouse with a 2-3% isofluorane/oxygen flow 5-10 min before injection, and during injection. Confirm anesthetization when animal is no longer ambulant and respirations have slowed by doing a toe pinch. Place the mouse on its back or side to access the tail vein. Use vet ointment on mouse's eyes to prevent drying while under anesthesia.
Wipe/spray the tail with 70% EtOH and inject 300 µl of LSPCs or PALETS onto the tail vein of the mouse using a 26 G insulin syringe. Start injecting distally into the tail vein and move proximally if the vein collapses or ruptures.
Place mouse in a clean cage until it maintains sternal recumbency. Do not place mouse with other cage mates until it has fully recovered. Mice should recover in 5 to 15 min. They can be placed on a heating pad or under a heat lamp to maintain body temperature if recovery takes longer. Mice should be monitored closely to safeguard against over-heating.
Monitor the animal several hours after injection to ensure anesthesia wears off, and there are no ill effects of the treatment. Allow the LSPCs or PALETS to circulate for at least 24 hr.
4. Analysis of Protein Expression via Flow Cytometry
Euthanize mice with a 20% CO2 flow rate for 15 min.
Dissect out half a hemisphere of brain by cutting away the skin and skull of the mouse using scissors. Peel away half a hemisphere of brain away from the skull using tongs or the end of a pair of scissors.
Press half a hemisphere of brain from each mouse through a 40 µm mesh cell strainer using 2.5 ml of FACS buffer (1x PBS, 1% fetal bovine serum, 10 mM EDTA) in a Petri dish with the plunger of a syringe.
Rinse the cell strainer and Petri dish with an additional 2.5 ml of FACS buffer. Put the single cell suspension in a 15 ml conical tube on ice.
Pipet 100 µl of the 5 ml single cell suspension into a 1.5 ml microcentrifuge tube and centrifuge for 5 min at 350 x g. Discard the supernatant and resuspend cell pellet in 1 ml of FACS buffer. Spin at 350 x g for 5 min. Repeat with another 1 ml of FACS buffer. Keep the cell suspension on ice.
Incubate the cells in 100 µl of 1:100 dilution of a 0.5 mg/ml rat anti-mouse Fc block in FACS buffer for 30-60 min on ice. Pellet and wash the cells as in step 4.5. Keep the cell suspension on ice.
Incubate the cells in 100 µl of a 20 µg/ml fluorescent antibody against mouse PrPC in 7% mouse serum in FACS buffer on ice for 30-60 min. Pellet and wash the cells as in step 4.5. Keep the cell suspension on ice.
Pellet and re-suspend the cells in 1 ml of RBC lysis buffer (1x PBS, 155 mM NH4Cl, 12 mM NaHCO3, 0.1 mM EDTA) for 1 min. Pellet as in step 4.5 and resuspend the cells in 1 ml of FACS buffer. Keep the cell suspension on ice.
Analyze PrPC expression using a flow cytometer as described previously10. Gate live cells from total cell population. Gate PrPC + cells from live cell population to determine MFI (median fluorescent intensity).
Representative Results
To increase the efficiency of siRNA encapsulation within anionic PALETS, the siRNA was mixed with protamine. To determine the best protamine concentration for the siRNA, the siRNA was mixed with different concentrations of protamine, from 1:1 to 2:1 (Figure 3A). There was a 60-65% siRNA encapsulation efficiency in anionic liposomes without the use of protamine. Samples with protamine:siRNA molar ratios from 1:1 to 1.5:1 (133-266 nM) had 80-90% siRNA encapsulation. Molar ratios above 1.5:1 resulted in precipitation of siRNA/protamine complexes. These precipitated complexes were not encapsulated into anionic liposomes. After the addition of protamine to the siRNA there was a slight drop in the concentration of the siRNA, due to the dilution; however, the concentration remained stable after the addition of protamine (Figure 3B). After filtering the liposomes with 50 kDa filters, 90-95% of the siRNA was associated with the liposome retentate, while 5-10% of the siRNA was 'free' in the filtrate. No siRNA was detected in protamine or liposome only samples (Figure 3C).
To determine the effect of LSPCs in vivo, wildtype mice were treated with LSPCs for 24 hr. PrPC expression levels of wildtype mice treated with LSPCs were compared to mice treated with 1x PBS and to PrP knockout (PrPKO) mice (Figure 4). Flow cytometric analysis of PrPC in the brains of mice treated with LSPCs showed a decrease in PrPC levels (Figure 4A and 4B). In one experiment, all mice had 80-90% reduction of PrPC levels, close to PrPC levels that were observed in the PrPKO mice (Figure 4A). Mice treated with LSPCs in two more independent experiments showed a 40-80% reduction of PrPC levels (Figure 4B). Out of the total LSPCs treated mice (n = 14), there was only one mouse had no response to the LSPCs.
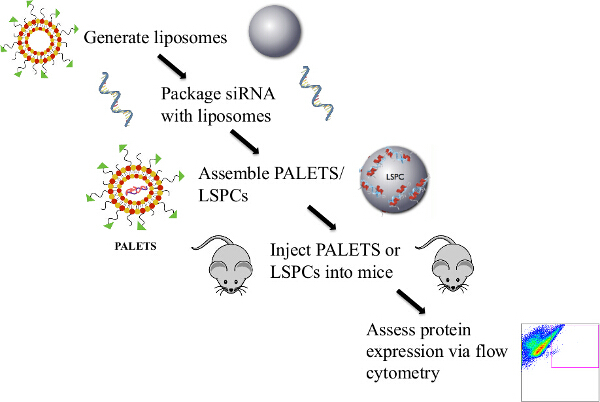 Figure 1: Overview of the Protocol to Generate PALETS/LSPCs and Deliver siRNA Intravenously to the CNS of Mice. Liposomes were generated via the thin lipid film hydration method. The PrPC siRNA was then attached to the liposomes either through an electrostatic interaction or by encapsulation. The PALETS or LSPCs were completed by the addition of the CNS targeting peptide RVG-9r. After assembly, the PALETS/LSPCs were injected into the tail veins of mice, and 24 hr after treatment PrPC expression was assessed via flow cytometry. Please click here to view a larger version of this figure.
Figure 1: Overview of the Protocol to Generate PALETS/LSPCs and Deliver siRNA Intravenously to the CNS of Mice. Liposomes were generated via the thin lipid film hydration method. The PrPC siRNA was then attached to the liposomes either through an electrostatic interaction or by encapsulation. The PALETS or LSPCs were completed by the addition of the CNS targeting peptide RVG-9r. After assembly, the PALETS/LSPCs were injected into the tail veins of mice, and 24 hr after treatment PrPC expression was assessed via flow cytometry. Please click here to view a larger version of this figure.
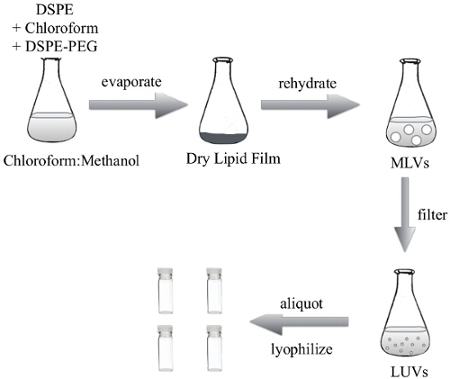 Figure 2: Protocol of the Thin lipid Film Hydration Method to Generate DSPE Liposomes for the PALETS Delivery Vehicle. The lipids were dissolved and mixed in a chloroform:methanol solution, which was evaporated to generate a dry lipid film. The dry lipid film was resuspended in 1x PBS to create multilamellar vesicles (MLVs). MLVs were then uniformly sized using an extruder to generate LUVs. The LUVs can then be used in suspension, or lyophilized in single use aliquots. Please click here to view a larger version of this figure.
Figure 2: Protocol of the Thin lipid Film Hydration Method to Generate DSPE Liposomes for the PALETS Delivery Vehicle. The lipids were dissolved and mixed in a chloroform:methanol solution, which was evaporated to generate a dry lipid film. The dry lipid film was resuspended in 1x PBS to create multilamellar vesicles (MLVs). MLVs were then uniformly sized using an extruder to generate LUVs. The LUVs can then be used in suspension, or lyophilized in single use aliquots. Please click here to view a larger version of this figure.
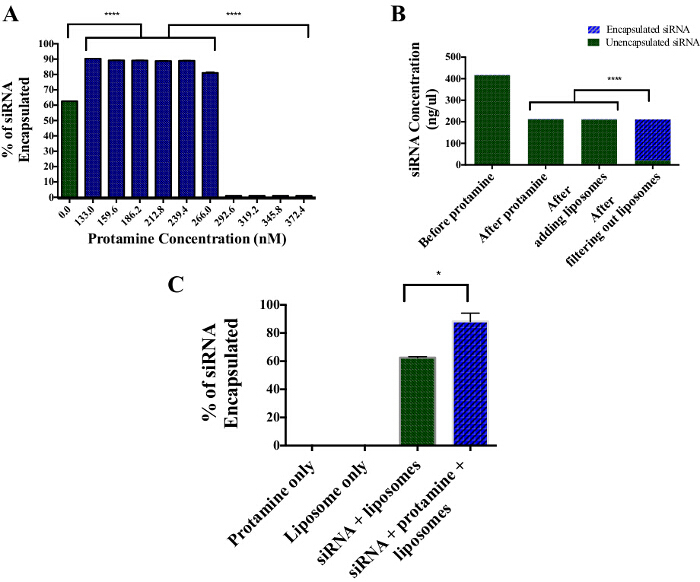 Figure 3:Encapsulation Efficiency of siRNA into DSPE PALETS using Protamine Sulfate. (A) About 60-65% of the siRNA was encapsulated without the addition of protamine. With the addition of protamine, there was a 90% encapsulation efficiency between 1:1 and 1.5:1 protamine:siRNA ratio. (B) After filtering out the liposomes from any free siRNA, 90% of the siRNA was found in the liposomal fraction, whereas 5-10% of unencapsulated siRNA was found in the filtrate. (C) Protamine and liposome only solutions are free of encapsulated siRNA. About 60-65% of siRNA was encapsulated within DSPE liposomes without the use of protamine. With the use of protamine at a concentration of 186.2 nM, about 90% of the siRNA was encapsulated within the DSPE liposomes. Error bars indicate SEM. **** indicates P <0.0001. * indicates P <0.01. Please click here to view a larger version of this figure.
Figure 3:Encapsulation Efficiency of siRNA into DSPE PALETS using Protamine Sulfate. (A) About 60-65% of the siRNA was encapsulated without the addition of protamine. With the addition of protamine, there was a 90% encapsulation efficiency between 1:1 and 1.5:1 protamine:siRNA ratio. (B) After filtering out the liposomes from any free siRNA, 90% of the siRNA was found in the liposomal fraction, whereas 5-10% of unencapsulated siRNA was found in the filtrate. (C) Protamine and liposome only solutions are free of encapsulated siRNA. About 60-65% of siRNA was encapsulated within DSPE liposomes without the use of protamine. With the use of protamine at a concentration of 186.2 nM, about 90% of the siRNA was encapsulated within the DSPE liposomes. Error bars indicate SEM. **** indicates P <0.0001. * indicates P <0.01. Please click here to view a larger version of this figure.
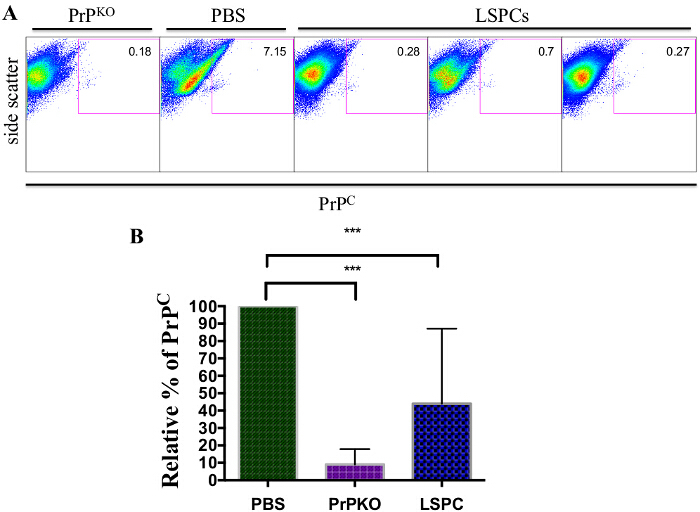 Figure 4:Representative Flow Cytometric Analysis of Brain Cell Suspensions 24 hr after Treatment with LSPCs. LSPCs were injected into the tail veins of wildtype mice and brains were harvested 24 hr later. PBS was used as a treatment control, and a PrPKO mouse was used as a control for PrPC levels. (A) Mice treated with LSPCs showed a significant decrease of PrPC levels compared to the PBS control, which shows wildtype PrPC levels. The LSPCs treated mice showed PrPC levels close to that of a PrPKO mouse. (B) Cumulative data from three independent experiments using the same 24 hr treatment protocol showed the relative amount of PrPC in PBS wildtype and LSPCs treated mice. Across the three experiments, mice treated with LSPCs showed significant decreases in PrPC levels as compared to PBS wildtype controls. Error bars indicate SEM. *** indicates P <0.0003. Please click here to view a larger version of this figure.
Figure 4:Representative Flow Cytometric Analysis of Brain Cell Suspensions 24 hr after Treatment with LSPCs. LSPCs were injected into the tail veins of wildtype mice and brains were harvested 24 hr later. PBS was used as a treatment control, and a PrPKO mouse was used as a control for PrPC levels. (A) Mice treated with LSPCs showed a significant decrease of PrPC levels compared to the PBS control, which shows wildtype PrPC levels. The LSPCs treated mice showed PrPC levels close to that of a PrPKO mouse. (B) Cumulative data from three independent experiments using the same 24 hr treatment protocol showed the relative amount of PrPC in PBS wildtype and LSPCs treated mice. Across the three experiments, mice treated with LSPCs showed significant decreases in PrPC levels as compared to PBS wildtype controls. Error bars indicate SEM. *** indicates P <0.0003. Please click here to view a larger version of this figure.
Discussion
This report describes a protocol to create two targeted delivery systems that efficiently transports siRNA to the CNS. Previous methods of delivering siRNA to the CNS included injecting siRNA/shRNA vectors directly into the brain, intravenous injection of targeted siRNA, or intravenous injection of non-targeted liposome-siRNA complexes. Injection of siRNA/shRNA vectors into the CNS does cause a decrease in target protein expression levels. However, the siRNA/shRNA does not diffuse freely through the CNS. Furthermore, these injections result in damage to the adjacent nervous tissue6-9. The intravenous injection of targeted siRNA also results in reduction of the target protein. However, most of the siRNA is degraded by serum proteins20. Lastly, intravenous injection of untargeted liposome-siRNA complexes results in the entrapment of the siRNA within the liver and degradation by the mononuclear phagocyte system21. The siRNA delivery vehicles described above, LSPCs and PALETS, provide a safer, more effective, and efficient delivery system than previous methods by delivering siRNA directly to the CNS. The LSPCs and the PALETS are also capable of transporting other small molecule drugs to the CNS.
PALETS or LSPCs should be used within a few hours after assembly. Otherwise there is a risk of the siRNA becoming non-functional/degraded. The other critical step that cannot be stopped/interrupted is the preparation and running of the cell suspension for flow cytometry. It is important to perform flow cytometry on the same day that the cell suspension is prepared, since the suspension contains live cells that need to be alive for analysis.
There are a few steps within the protocol that can be altered depending on whether the LSPCs or PALETS will be used in vitro or in vivo, on what small molecule drug is loaded into the LSPCs or PALETS, and on laboratory preference. First,the lipid molar ratios of the LSPCs and PALETS can be optimized for other applications. However, phosphatidylethanolamine (PE in DSPE) hydrates poorly when used at a 60% weight/weight or higher. When used at these higher concentrations, DSPE molecules will aggregate with each other and form an insoluble mass of lipids. This mass of lipids is not able to encapsulate siRNA. If N2 gas is unavailable, the lipid mix can be dried using evaporation. Evaporation takes about 3 days, and should be performed in a fume hood due to the use of methanol and chloroform. In this protocol, the thin lipid film was resuspended with 1x PBS but it can also be resuspended with other hydration buffers. Other buffers include water, 10% sucrose, or any other aqueous buffer. The choice of rehydration buffer is dependent on the intended use of the liposomes and the agent being encapsulated within the liposome22,23. The hydration buffer should be kept above the gel-liquid crystal transition temperature (Tc or Tm) of the lipids or the liposomes will not rehydrate properly. In the described protocol, the suspended liposomes were uniformly sized using an extruder. However, the liposomes can also be sized using syringe filters or sonication. The same filter pore sizes used for the extruder can be used as syringe filters. Also, the liposome suspension can be resized after hydration with the siRNA solution. However, we observed a loss of siRNA from liposomes when sized by the extruder after the hydration step (unpublished data).
Since the biological role of PrPC is not yet understood5, it is possible that there could be some deleterious effects produced by reducing PrPC levels. However, because prion protein deficient mice develop, behave, and age normally24, it is likely that these deleterious effects would either be minor or not visibly apparent. Furthermore, siRNA knockdown of PrPC expression would not completely abolish protein expression and is completely reversible with no possibility of viral oncogenesis, unlike lentivector RNAi23.
Limitations with the delivery systems include reduced uptake of anionic PALETS into biological membranes, and the immunogenicity of LSPCs due to the cationic lipids. If immunogenicity of the LSPCs is observed, PALETS can be used as an alternative. Reduced uptake of anionic PALETS into anionic biologic membranes can be overcome by using cationic PALETS, or by reformulating anionic PALETS with a mixture of cationic and anionic lipids.
In this report, the reduction of PrPC varies widely from mouse to mouse. This variation could be due to a variety of factors: variation between mice, the small diameter of veins in mice, or degradation of siRNA. The use of inbred mice should eliminate much of the variability between mice, but variation is always possible even with inbred mice. Also, mice have very small veins compared to other animal models. So, injecting a large volume into the veins of mice can be problematic. We recommend practicing injections before trying to inject PALETS or LSPCs. However, even with practice, sometimes it still takes a couple of injections to get all the volume of PALETS/LSPCs into the mouse, and the vein can collapse at any one of these injections. So, one mouse might receive all the volume of PALETS/LSPCs, whereas another mouse might only receive 75-90% of the PALETS/LSPCs volume.
Other possible sources of variation include circulation time and degradation of the siRNA by serum proteins. The PALETS/LSPCS might circulate longer before reaching the brain in one mouse compared to another, which could explain the variation. We only allowed LSPCs to circulate for 24 hr before euthanizing mice in the above experiment. If the circulation time is causing the variation, then increasing the circulation time beyond 24 hr should reduce some variation. Even though we package the siRNA with the PALETS/LSPCs, there is always a chance of degradation of the siRNA and/or the delivery vehicles. As mentioned earlier, the cationic lipids are slightly immunogenic. So, if immune cells within the bloodstream are attacking the vehicles, then it would take longer for the vehicles to be delivered to the brain or the vehicles might become degraded. Switching from cationic lipids to anionic PALETS might help reduce the slow circulation time or degradation when using cationic lipids.
The RVG-9r peptide transports the LSPCs and PALETS to any cell within the CNS that contains nicotinic acetylcholine receptors. This includes both neuronal cells microglial cells and astrocytes26. For PALETS or LSPCs to be an effective treatment for prion diseases it is important to target all cells that contribute to prion pathogenesis. Neuronal cells contain the most PrPC of any other cell type within the CNS. Therefore, neuronal cells help to contribute the spread of prions throughout the brain27,28. Targeting neuronal cells and reducing the cells' PrPC expression could result in a decrease of prion pathogenesis. However, neuronal cells are not the only cell type that contributes to prion disease. Astrocytes have been implicated in the replication of prions29,30. Therefore, not only is it important to target neuronal cells, but to also target astrocytes to reduce prion pathogenesis.
The delivery systems described here are amenable to various optimization strategies that would make them suitable for a wide range of disease applications. LSPCs and PALETS are capable of transporting any small molecule drug to the CNS, not just siRNA, which gives the vehicles the potential to treat more than just neurodegenerative diseases. PALETS and LSPCs could be used to treat any disease that affects the brain depending on the small molecule drug loaded into the vehicles. Also, changing the targeting peptide could allow the delivery vehicles to deliver small molecule drugs to a specific subset of cells within the CNS, instead of any cell that has acetylcholine receptors. PALETS and LSPCs represent a novel, more efficient and flexible delivery system for small molecule drugs to treat diseases that affect the CNS.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We would like to acknowledge the following funding sources: the CSU Infectious Disease Translational Research Training Program (ID:TRTP) and the NIH research grant program (R01 NS075214-01A1). We would like to thank the Telling lab for the use of their monoclonal antibody PRC5. We would also like to thank the Dow lab for DOTAP liposomes, and for sharing their expertise in generating liposomes.
References
- Bolton DC, Mckinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Pruisner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Pruisner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Jeffrey M, McGovern G, Sisò S, González L. Cellular and sub-cellular pathology of prion diseases: relationship between morphological changes, accumulation of abnormal prion protein and clinical disease. Acta Neuropathologica. 2011;121(1):113–134. doi: 10.1007/s00401-010-0700-3. [DOI] [PubMed] [Google Scholar]
- Büeler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, et al. Lentivector-mediated RNAi efficiently suppresses prion protein and prolongs survival of scrapie-infected mice. J Clin Invest. 2006;116(12):3204–3210. doi: 10.1172/JCI29236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M, Farmer M, Mirabile I, Brandner S, Collinge J, Mallucci G. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice. Proc Natl Acad Sci. 2008;105(29):10238–10243. doi: 10.1073/pnas.0802759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallozzi M, et al. Prnp knockdown in transgenic mice using RNA interference. Transgenic Research. 2008;17(5):783–791. doi: 10.1007/s11248-008-9179-2. [DOI] [PubMed] [Google Scholar]
- Mallucci G, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53(3):325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Pulford B, et al. Liposome-siRNA-peptide complexes cross the blood-brain barrier and significantly decrease PrPC on neuronal cells PrPRes in infected cultures. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol. 1997;15:167–173. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- Freimark BD, et al. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998;160(9):4580–4586. [PubMed] [Google Scholar]
- Balzas DA, Godbey WT. Liposomes for use in gene delivery. J Drug Deliv. 2011. [DOI] [PMC free article] [PubMed]
- Srinivasan C, Burgess DJ. Optimization and characterization of anionic lipoplexes for gene delivery. J Control Release. 2009;136(1):62–70. doi: 10.1016/j.jconrel.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Lakkaruju A, Dubinsky JM, Low WC, Rahman Y. Neurons are protected from excitotoxic death by p53 antisense oligonucleotides delivered in anionic liposomes. J Biol Chem. 2001;276(34):32000–32007. doi: 10.1074/jbc.M100138200. [DOI] [PubMed] [Google Scholar]
- Tao Y, Han J, Dou H. Brain-targeting gene delivery using a rabies virus glycoprotein peptide modulated hollow liposomes: bio-behavioral study. J Mater Chem. 2012;22:11808–11815. [Google Scholar]
- Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4(9):891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- Li S, Rizzo MA, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5(7):930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- Kumar P, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Voinea M, Simionescu M. Designing of 'intelligent' liposomes for efficient delivery of drugs. J Cell Mol Med. 2002;6(4):465–474. doi: 10.1111/j.1582-4934.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, et al. Neurons are protected from excitotoxic death by p53 antisense oligonucleotides delivered in anionic liposomes. J. Biol. Chem. 2001;276(34):32000–32007. doi: 10.1074/jbc.M100138200. [DOI] [PubMed] [Google Scholar]
- Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4(9):891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- Büeler H, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn D, Novina C, Sharp P. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Bio. 2003;4(6):457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- Graham A, Ray M, Perry E, Jaros E. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J Chem Neuroanat. 2003;25(2):97–113. doi: 10.1016/s0891-0618(02)00100-x. [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, Prusiner SB, Stowring LE. Scrapie prion proteins are synthesized in neurons. Am J Pathol. 1986;122(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Moser M, Colello R, Pott U, Oesch B. Developmental expression of the prion protein gene in glial cells. Neuron. 1995;14(3):509–517. doi: 10.1016/0896-6273(95)90307-0. [DOI] [PubMed] [Google Scholar]
- Cronier S, Laude H, Peyrin JM. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc Natl Acad Sci. 2004;101(33):12271–12276. doi: 10.1073/pnas.0402725101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich J, Bendheim P, Kim Y. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc Natl Acad Sci. 1991;88(2):375–379. doi: 10.1073/pnas.88.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]


