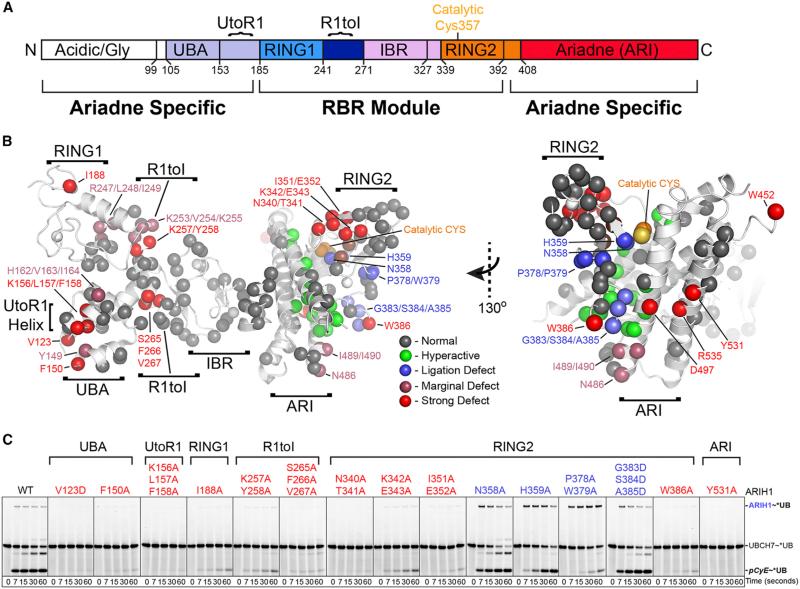
Figure 4. Broad Mutational Survey Reveals Numerous ARIH1 Surfaces Contribute to CRL Substrate Ubiquitylation.
(A) ARIH1 domains.
(B) Sites of Ala mutations as spheres on structure of autoinhibited ARIH1 (Duda et al., 2013), colored by effect on *UB transfer from UBCH7 via ARIH1/neddylated CRL1FBW7ΔD to pCyE (in Figure S4): gray, normal; green, hyperactive; blue, ligation defect/accumulation of normally transient thioester-bonded ARIH1~UB intermediate; raspberry, marginal defect; red, strong defect.
(C) Most defective (red) and ligation-impaired (blue) ARIH1 Ala scan mutants in pulse-chase assays monitoring entire pathway: *UB transfer from UBCH7 via the indicated ARIH1 mutants and neddylated CRL1FBW7ΔD to pCyE. Ligation-impaired mutants are scored by appearance and dissipation of ARIH1~UB thioester intermediate.