Abstract
High dietary protein has been hypothesized to cause lower bone mineral density (BMD) and greater fracture risk. Previous results are conflicting and few studies have assessed potential differences related to differing protein sources.
Objective
To determine associations between total protein intake, and protein intake by source (dairy, non-dairy animal, plant) with BMD, BMD change, and incident osteoporotic fracture.
Design/Setting
Prospective cohort study (Canadian Multicentre Osteoporosis Study).
Participants/Measures
Protein intake was assessed as percent of total energy intake (TEI) at Year 2 (1997–99) using a food frequency questionnaire (N=6510). Participants were contacted annually to ascertain incident fracture. Total hip and lumbar spine BMD was measured at baseline and Year 5. Analyses were stratified by group (men 25–49 y, men 50+ y, premenopausal women 25–49 y, and postmenopausal women 50+ y) and adjusted for major confounders. Fracture analyses were limited to those 50+ y.
Results
Intakes of dairy protein (with adjustment for BMI) were positively associated with total hip BMD among men and women aged 50+ y, and in men aged 25–49. Among adults aged 50+ y, those with protein intakes of <12% TEI (women) and <11% TEI (men) had increased fracture risk compared to those with intakes of 15% TEI. Fracture risk did not significantly change as intake increased above 15% TEI, and was not significantly associated with protein source.
Conclusions
In contrast to hypothesized risk of high protein, we found that for adults 50+ y, low protein intake (below 15% TEI) may lead to increased fracture risk. Source of protein was a determinant of BMD, but not fracture risk.
Keywords: Protein intake, bone mineral density, osteoporosis, fracture, cohort study
Introduction
Fractures, especially fragility fractures resulting from low trauma, are the primary health risk of osteoporosis (1). Current guidelines for protein intakes among adults specified in the Institute of Medicine Dietary Reference Intakes include a Recommended Dietary Allowance (RDA) of 0.8 g/kg based on maintenance of nitrogen balance, and an Acceptable Macronutrient Distribution Range of 10–35% of total energy intake (TEI), which recognizes the role of protein as an energy source (2). Maintenance of muscle and bone strength across the lifespan was not specifically considered as an endpoint for establishing protein requirements. High dietary protein has been associated with increased urinary calcium (3), and this was hypothesized to be the result of a high dietary acid load causing increased bone resorption. However, careful calcium balance studies have not shown that bone is the source of this calcium since calcium absorption may also increase (4). In fact, a recent review concluded that the dietary acid load hypothesis was not supported by the evidence (5).
There have been several large cohort studies assessing the cross-sectional association between protein intake and bone mineral density (BMD) (6–8). The balance of evidence from both small and large cross-sectional studies as assessed in a meta-analysis of Darling et al. (9) suggests there is either no association or a small positive association between protein intakes and BMD. Among the two longitudinal studies with larger sample sizes, Hannan et al. (10) reported that low protein intake was associated with increased bone loss, but in contrast Rapuri et al. reported no statistically significant association (6). This heterogeneity of results could be related to source of the protein. In a large US study considering fracture outcomes in women, Feskanich et al. (11) found inconclusive evidence relating dietary protein (total, animal-based, plant-based) and hip fracture, i.e. higher protein could be associated with increased risk (at least 10% higher), similar risk (within 5% of null effect), or reduced risk (at least 10% lower). Meyer et al. (12) tested the association between animal protein intake and hip fracture in a large cohort of Norwegian men and women, and again found inconclusive results. A third study of Munger et al. (13) found that both higher animal protein and higher total protein were associated with lower risk of hip fracture while vegetable protein was potentially associated with higher risk of hip fracture. The study of Feskanich et al. (11) also considered forearm fracture and found that higher animal protein and higher total protein were associated with increased risk of forearm fracture, a fracture much more likely in their younger (35–59 y) cohort. Finally the recent work of Sahni et al. (14) addressed a missing element in the literature, namely the hypothesized interaction of calcium and protein (15), but this study should be viewed as suggestive due to low total number of fractures.
The purpose of this analysis was to determine the associations between total protein intake and protein intake by food source (dairy, non-dairy animal, plant) with BMD among men and women aged 25 y and older, and incident fracture risk among men and postmenopausal women aged 50 y and older.
Methods
Setting
The study setting was the Canadian Multicentre Osteoporosis Study (CaMos), an on-going prospective cohort study recruited from 1995–97 (www.camos.org). Households were randomly selected from a list of residential phone numbers within a 50-kilometer radius of one of nine Canadian cities and participants were randomly selected from eligible household members (age 25 years and older) using a standardized protocol. Of all those randomly selected, 42% agreed to full participation and completed a standardized interviewer-administered questionnaire (CaMos questionnaire ©1995) at baseline. The questionnaire, designed to capture detailed information about risk factors for fracture, assessed demographics, general health, nutrition, reproduction, medication use and medical history. Supplement and medication use was derived from an inventory of prescriptions and containers brought to the interview. The baseline interview also included anthropometrics, BMD and spine radiographs. Height was measured without shoes, using a height rod mounted on beam balance scale, a wall-mounted stadiometer, or a ruler on the wall. Weight was measured in light clothing using a beam balance or electronic scale. Body mass index (BMI) was calculated as weight(kg)/height(m)2. Ethics approval was granted through McGill University and the appropriate ethics review boards for each participating center. All participants gave written informed consent in accordance with the Helsinki declaration.
Study sample
Participants from CaMos were eligible for the present analysis if the Year 2 (1997–99) food frequency questionnaire had fewer than 10 missing responses (N=6510), with exclusions detailed in Figure 1. Those with extreme energy intake (<500 or > 5000 kcal/d) were excluded from the regression analyses (16). For Year 5 (2000–02) BMD outcomes, separate analyses were performed by sex and age-group (25–49 y and 50+ y at baseline), and excluded users of oral/intravenous corticosteroids. We restricted the analyses in women to premenopausal women 25–49 y and postmenopausal women 50+ y, as menopausal status is one of the strongest determinants of bone loss (17). Due to low fracture incidence in the younger cohorts, the analysis for incident fracture included postmenopausal women aged 50+ y and men aged 50+ y at baseline. The fracture analyses excluded those who had fracture between baseline and Year 2, or discontinued the study after Year 2.
Figure 1.
Flowchart detailing Canadian Multicentre Osteoporosis Study participants with exposure assessment at Year 2, exclusions from the regression analyses for BMD and fracture outcomes,and study sample size for each outcome
Food Frequency Questionnaire (FFQ)
The FFQ used in CaMos for the Year 2 follow-up was derived from items on the short form Block questionnaire (18) with modifications according to the Canadian diet. A standard portion size was specified with frequency ranging from never/ less than once a month to 6 or more times per day. The main variables used in this study were derived from the food and beverage portion of the questionnaire; there were 51 food items and 18 beverage items. Total energy intake (TEI) and protein intake were calculated by using the frequency and specified portion size from the questionnaire together with content information from the Canadian Nutrient File (19). Where multiple foods were listed in a single question, we used the mean value for all listed foods. Protein intake was divided by source: dairy, non-dairy animal (meat, poultry, eggs, fish), and plant-based (grains, legumes, nuts, vegetables).
Bone mineral density
BMD was measured at the lumbar spine (L1–L4) and total hip by dual energy x-ray absorptiometry. At baseline seven centers had Hologic densitometers and two centers had Lunar densitometers. The same machine was used at baseline and Year 5 in all centers. All Lunar measurements were converted to equivalent Hologic values using standard reference formulas (20). Daily quality control to assess for longitudinal drift at each center was done by scanning a local machine-specific phantom. The threshold for longitudinal change was defined as a 1% difference from the calibration point and seven of the nine machines remained within the limits; Hamilton and Toronto both needed a one-time correction in longitudinal calibration. Machines were also cross-calibrated using measurements from an anthropomorphic phantom that was circulated between centers during each measurement cycle.
Fracture assessment
Self-reported incident clinical fractures were identified by annual postal questionnaire up to Year 15 or by interviewer-administered questionnaires at the scheduled interviews (in the 3rd, 5th and 10th years after study entry). Further information concerning each fracture was gathered using a structured interview that included the date, fracture site, circumstances leading to the fracture and its management. Participants who reported fractures were asked for consent to contact the treating physician or hospital for further confirmation.
All incident clinical fractures between the FFQ (Year 2, 1997–99) and Year 15 follow-up (September 2012) were included, with the exception of fractures of the skull, face, hands, ankles, and feet (as is customary in osteoporosis-related fracture analysis). Fragility (low-trauma) fractures are those involving trauma less than or equivalent to fall from standing height. Main fracture refers to the four fracture sites designated by the World Health Organization (hip, forearm, clinical spine and humerus) (21).
Statistical methods
Missing responses on the FFQ were imputed using median intake. We used imputed values for 1.3% (5655/449,190) of the FFQ responses. In sensitivity analysis, we compared imputation of the median intake vs. imputation of no intake for missing values and found a very high correspondence for total calories, total protein and protein by source (Pearson r=0.999). Multivariate linear regression models were used to examine the association between protein intake (as a percentage of TEI) with the Year 5 BMD and with BMD change over time (ΔBMD = Year 5 value – baseline value) as outcomes. Multivariate Cox regression models were used to examine the association between the protein intake (as a percentage of TEI) with the incident fragility fractures and incident main fractures. Person-time for this analysis included the period from entry (Year 2) to exit time (earliest date of: incident fracture, death, loss to follow up or end of the study period). The proportional hazards assumption was assessed. All continuous covariates were assessed for non-linearity. There was a non-linear relationship between protein intake and incident fracture, notable when using both quartiles (pre-specified) and a restricted cubic spline model. We supplemented the usual strategy of using quartiles to account for non-linearity with spline models since spline models do not involve loss of information and therefore can provide a more detailed picture of the non-linear association.
Models were stratified by sex and age, and age was included as a covariate in the linear regression models and as the main time axis in the Cox model. To further control for confounding, we included a priori specified covariates in the final model (sample size permitting) in a complete case analysis. The specified covariates were TEI, study center education, height, smoking, alcohol intake, physical activity, sedentary hours, calcium and vitamin D supplement use, diagnosis of osteoporosis, bisphosphonate use, and hormone therapy (postmenopausal women). Regression analyses for BMD were repeated both with/without BMI as a covariate. In addition to the main protein analyses, separate models for all outcomes were run testing heterogeneity by protein source (dairy vs. non-dairy, plant vs. animal) as well as the protein source specific association (dairy, non-dairy animal, plant). For missing data, we performed sensitivity analyses for BMD outcomes comparing exposures among those in a specified category (died, no Year 5 interview, missing BMD, missing covariates) vs. the remaining sample and found no statistically significant between-group differences for any of TEI, total protein intake or protein intake by source. We also assessed fracture outcomes among those present at Year 2 with and without exposure assessment and with and without complete covariates and found that the missing data was not associated with age-adjusted fracture risk. All analyses were performed with Stata (Version 12) (College Station, Texas, USA).
Results
Table 1 shows the median and interquartile ranges of all the exposure parameters (TEI, total protein, protein intake by category, and conversion to %TEI) overall and by sex and age strata. The median daily energy intake was 1615 kcal overall, with medians of 56.9 g, 11.6 g, 17.6 g and 24.3 g of protein from all sources, dairy sources, non-dairy animal sources, and plant sources, respectively. There were small but statistically significant differences in the distribution of exposure variables by sex with men having slightly higher TEI, total protein intake, non-dairy animal protein intake, and plant protein intake, but slightly lower dairy protein intake than women. However, when expressed as %TEI, women had higher intakes of total, dairy and plant-based protein compared to men. Those younger than 50 years of age at baseline had slightly lower total protein, dairy protein, and plant protein intake but higher non-dairy protein intake than those 50 years and older. The median protein intake by body weight was 0.79 g/kg per day, and there were significant differences by age and sex with higher protein intake by body weight among women vs. men and among those 50 years and older vs. those who were younger.
Table 1.
Median and Interquartile Range of Daily Dietary Protein Intake derived from the Year 2 Food Frequency Questionnaire (FFQ) bySex and Age Group
| Total sample N=6510 | Men N=1919 | Women N=4591 | Age < 50 y N=1248 | Age ≥ 50 y N=5262 | |
|---|---|---|---|---|---|
| Total Energy Intake [TEI] (kcal) | 1615 1291–1986 | 1715* 1364–2123 | 1579* 1267–1923 | 1588 1265–1980 | 1618 1300–1987 |
| Total Protein Intake [TPI](g) | 56.9 44.5–70.7 | 57.8* 44.9–72.2 | 56.5* 44.3–70.0 | 55.0* 42.7–68.7 | 57.4* 45.0–71.0 |
| TPI per weight (g/kg) | 0.79 0.60–1.03 | 0.70* 0.55–0.91 | 0.82* 0.63–1.07 | 0.75* 0.57–1.00 | 0.80* 0.61–1.03 |
| Dairy Protein Intake (g) | 11.6 6.5–21.7 | 10.8* 6.1–20.5 | 11.9* 6.9–22.3 | 11.2* 6.2–20.5 | 11.6* 6.7–22.1 |
| Non-dairy Animal Protein Intake (g) | 17.6 12.8–23.0 | 18.5* 13.6–24.8 | 17.2* 12.5–22.5 | 18.0* 12.9–23.6 | 17.4* 12.8–22.9 |
| Plant-based Protein Intake (g) | 24.3 18.8–31.0 | 25.3* 19.0–32.5 | 24.0* 18.7–30.4 | 22.7* 17.3–30.0 | 24.7* 19.2–31.2 |
| Protein as %TEI | 14.1 12.6–15.7 | 13.6* 12.0–15.1 | 14.3* 12.8–15.9 | 14.0* 12.4–15.5 | 14.1* 12.6–15.7 |
| Dairy Protein as %TEI | 3.0 1.8–4.8 | 2.5* 1.5–4.2 | 3.2* 1.9–5.0 | 2.9 1.6–4.6 | 3.0 1.8–4.8 |
| Non-dairy Animal Protein as %TEI | 4.4 3.3–5.6 | 4.4 3.3–5.6 | 4.3 3.3–5.6 | 4.5* 3.4–5.9 | 4.3* 3.3–5.5 |
| Plant-based Protein as %TEI | 6.1 5.3–7.0 | 6.0* 5.1–6.9 | 6.2* 5.4–7.0 | 5.8* 4.9–6.7 | 6.2* 5.4–7.0 |
Between group (men vs. women, age < 50 y vs. age ≥ 50 y) differences with rank sum test p < 0.05 are indicated by *.
Cross-sectional BMD
Models with Year 5 BMD as the main outcome all demonstrated statistically significant heterogeneity by protein source (plant vs. animal sources or dairy vs. non-dairy); therefore all results are presented with protein intake by source. Table 2 shows the associations between protein intake by source and total hip and lumbar spine BMD after adjustment for potential confounders. There were positive associations between dairy protein intake and total hip BMD, which were statistically significant among men and postmenopausal women 50+ y old and having the same direction among younger participants; there were negative associations between plant-based protein and total hip BMD that were statistically significant only among women, but again having the same direction in all subgroups. There were no statistically significant associations between non-dairy animal protein intake and total hip BMD.
Table 2.
The Association between Protein Intake (Dairy, Non-dairy Animal, and Plant) as percent of Total Energy Intake (%TEI) and Year5 BMD (Hip and Lumbar Spine) and Change in BMD between Baseline and Year 5 (Total Hip and Lumbar Spine)
| Estimated beta coefficient and 95% CI (g/cm 2) | Outcome | ||||
|---|---|---|---|---|---|
| Year 5 Hip BMD | 5-yr Hip BMD change | Year 5 Spine BMD | 5-yr Spine BMD change | ||
| Dairy Protein (% TEI) | Men 25–49 y | 0.013 −0.002, 0.028 | 0.001 −0.003, 0.005 | 0.020* 0.004, 0.035 | −0.002 −0.008, 0.004 |
| Premenopausal Women 25–49 y | 0.009 −0.001, 0.019 | 0.002 −0.001, 0.005 | 0.010 −0.002, 0.021 | 0.001 −0.003, 0.005 | |
| Men 50+ y | 0.015* 0.006, 0.023 | 0.001 −0.002, 0.003 | 0.014* 0.002, 0.025 | 0.002 −0.002, 0.005 | |
| Postmenopausal Women 50+ y | 0.006* 0.001, 0.010 | 0.002* 0.000, 0.003 | 0.002 −0.004, 0.008 | 0.001 −0.001, 0.003 | |
| Non-dairy Animal Protein (% TEI) | Men 25–49 y | −0.001 −0.016, 0.013 | 0.000 −0.004, 0.004 | −0.012 −0.026, 0.003 | −0.001 −0.006, 0.005 |
| Premenopausal Women 25–49 y | −0.004 −0.015, 0.006 | 0.001 −0.002, 0.005 | −0.012* −0.024, 0.000 | −0.001 −0.005, 0.003 | |
| Men 50+ y | −0.002 −0.011, 0.006 | 0.001 −0.001, 0.004 | 0.000 −0.011, 0.011 | 0.000 −0.003, 0.004 | |
| Postmenopausal Women 50+ y | 0.004 −0.001, 0.009 | 0.000 −0.002, 0.002 | 0.010* 0.003, 0.016 | 0.001 −0.001, 0.003 | |
| Plant Protein (% TEI) | Men 25–49 y | −0.010 −0.024, 0.003 | 0.001 −0.003, 0.005 | −0.013 −0.027, 0.001 | 0.000 −0.005, 0.005 |
| Premenopausal Women 25−49 y | −0.011* −0.022, −0.001 | −0.003 −0.006, 0.001 | −0.005 −0.017, 0.007 | −0.002 −0.006, 0.003 | |
| Men 50+ y | −0.007 −0.016, 0.001 | 0.001 −0.002, 0.003 | −0.009 −0.020, 0.002 | 0.001 −0.002, 0.005 | |
| Postmenopausal Women 50+ y | −0.006* −0.011, −0.001 | 0.000 −0.002, 0.002 | −0.012* −0.019, −0.005 | −0.003* −0.005, 0.000 | |
Data shown are regression coefficients and 95% CI. Regression analysis performed with standardized variables, dairy protein: 1 SD=2.3% TEI, non-dairy animal protein: 1 SD=1.9% TEI, plant protein: 1 SD=1.5% TEI. All models adjusted for age, height, TEI, center, education, smoking, alcohol intake, physical activity, sedentary hours, calcium and vitamin D supplement use, hormone therapy (women 50+ y), bisphosphonate use (50+ y), and diagnosis of osteoporosis (50+ y). The associations for which the 95% confidence interval excludes the null effect (p < 0.05) are indicated by an asterisk (*).
There were positive associations between dairy protein intake and lumbar spine BMD, which were statistically significant among men; there were negative associations between plant-based protein and lumbar spine BMD that were statistically significant only among postmenopausal women aged 50+ y. There was a negative association between non-dairy animal protein and lumbar spine BMD among women aged 25–49 y (statistically significant) with a similar association in younger men, while the reverse association was noted in older women. Figure 2 shows the association between protein intake by source and total hip and lumbar spine BMD after further adjustment for BMI. BMI-adjusted associations between plant protein intake and total hip BMD were attenuated and no longer statistically significant, while the positive associations between dairy protein and total hip BMD remained statistically significant. The adjustment for BMI had less effect at the spine, where all significant relationships remained after the adjustment except for the association between non-dairy animal protein and lumbar spine BMD among women aged 50+ y that was present without the adjustment for BMI.
Figure 2.
The Cross-sectional Association between Protein Intake (Dairy, Non-dairy Animal, Plant) as % TEI and Year 5 BMD (TotalHip and Lumbar Spine) adjusted for BMI
Data are shown as betas and error bars indicate the range of the 95% CI. Regression analysis done with standardized variables, dairy protein: 1 SD=2.3% TEI, non-dairy animal protein: 1 SD=1.9% TEI, plant protein: 1 SD=1.5% TEI. All models adjusted for age, height, TEI, center, education, smoking, alcohol intake, physical activity, sedentary hours, calcium and vitamin D supplement use, hormone therapy (women 50+ y), bisphosphonate use (50+ y), and diagnosis of osteoporosis (50+ y).
Longitudinal changes in BMI and BMD
We found statistically significant heterogeneity in one model with change in BMD from baseline to Year 5 as outcome and some evidence of heterogeneity among women in general (p < 0.10). Given that the cross-sectional models showed heterogeneity by protein source, we also considered protein intake by source in the longitudinal models (see Table 2). Higher dairy protein intake was associated with attenuated bone loss at the hip for postmenopausal women 50+ y old. The associations between dairy protein intake and change in total hip BMD in the other strata were similar but the 95% CI in these cases all included the null value. Higher plant protein was associated with greater bone loss at the lumbar spine for postmenopausal women 50+ y old, but with inconsistent results in other strata. There was no overall association between protein intake and BMI change and no heterogeneity by source. Therefore, while models for BMD change that included BMI change had improved model fit, the point estimates were minimally affected (data not shown).
Incident Fracture Risk
Between the Year 2 and Year 15 follow-up, there were 507 incident fragility fractures in 3202 women (mean 9.5 y, total 30,400 person years (p-y) follow-up) and 90 incident fragility fractures in 1341 men (mean 9.7 y, total 13,000 p-y follow-up). There were 437 incident main fractures in 3223 women (mean 9.6 y, total 31,000 p-y follow-up) and 81 incident main fractures in 1347 men (mean 9.7 y, total 13,000 p-y follow-up). Those with incident fracture were on average older and were more likely to have had a previous fragility fracture (data not shown).
Table 3 shows the association between quartile of protein intake and incident fracture in models adjusted only for age and in models including all covariates. There was no statistically significant heterogeneity by source of protein, and thus all results are presented only for total protein intake. Inclusion vs. exclusion of those with missing covariates had very little impact on the observed point estimates. The point estimates shifted slightly with adjustment for potential confounders in the full model. Women in the bottom quartile had the highest estimated risk of both fragility fracture and main fracture while women in the second from top quartile had the lowest risk. Men in the bottom quartile also had the highest risk of fragility fracture and main fracture, but the quartile with the lowest risk was less clear.
Table 3.
The Association between Quartiles of Protein Intake (as Percent of Total Energy Intake, %TEI) and Incident Fragility Fracture and Incident Main Fracture among Men 50+ y and Postmenopausal Women 50+ y
| Hazard Ratio 95% CI | Quartile Protein Intake | Fragility Fracture | Main Fracture | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Model 1 N=4786 | Model 2 N=4543 | Model 3 N=4543 | Model 1 N=4820 | Model 2 N=4570 | Model 3 N=4570 | ||
| Men | 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 | 0.72 0.43, 1.22 | 0.72 0.42,1.23 | 0.68 0.39, 1.18 | 0.64 0.36, 1.14 | 0.62 0.35, 1.11 | 0.57 0.32, 1.03 | |
| 3 | 0.65 0.37, 1.13 | 0.68 0.39,1.20 | 0.66 0.37, 1.18 | 0.74 0.42, 1.31 | 0.74 0.42, 1.31 | 0.70 0.39, 1.25 | |
| 4 | 0.72 0.40, 1.32 | 0.75 0.41,1.38 | 0.66 0.35, 1.24 | 0.66 0.34,1.29 | 0.65 0.33, 1.26 | 0.55 0.28, 1.09 | |
| Women | 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 | 0.88 0.69, 1.12 | 0.85 0.66, 1.09 | 0.81 0.63, 1.04 | 0.96 0.74,1.24 | 0.94 0.71, 1.23 | 0.90 0.68,1.18 | |
| 3 | 0.72* 0.56, 0.92 | 0.71* 0.55, 0.92 | 0.70* 0.54, 0.91 | 0.78 0.59,1.02 | 0.80 0.60,1.05 | 0.78 0.59, 1.04 | |
| 4 | 0.93 0.73, 1.17 | 0.93 0.73, 1.18 | 0.85 0.67, 1.09 | 0.95 0.74,1.23 | 0.97 0.74,1.26 | 0.90 0.69, 1.19 | |
Quartile protein intake: Cut-points 12.6%, 14.1%, 15.7% (as % TEI); Model 1: Age-adjusted model among eligible participants (regardless of missing covariates); Model 2: Age-adjusted model among the study sample (those with non-missing covariates); Model 3: adjusted for age, height, TEI, center (women only), education, smoking, alcohol intake, physical activity, sedentary hours, calcium and vitamin D supplement use, hormone therapy (women only), bisphosphonate use (women only), and diagnosis of osteoporosis (women only); The associations for which the 95% confidence interval excludes the null effect (p < 0.05) are indicated by an asterisk (*).
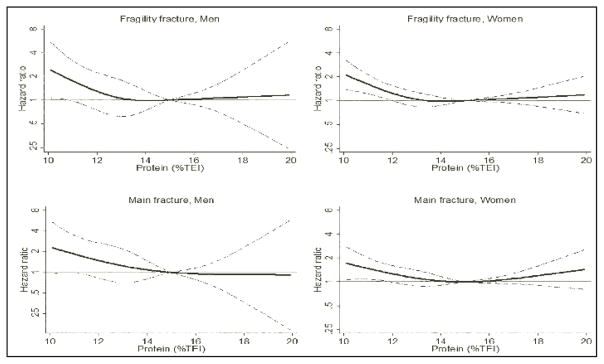
Figure 3 shows the non-linear relationship between protein intake as %TEI and incident fragility fracture as determined by cubic splines. Both men and women with protein intakes higher than 15% TEI had roughly similar fracture risk to those with intakes of 15% TEI. Men and women with intake below 15% TEI had increased risk in a dose response fashion with women <12% TEI and men <11% TEI having statistically significant increased risk compared to those with intakes of 15% TEI. Consideration of the four main osteoporotic fracture sites yielded non-linear associations very similar to those described above for incident fragility fractures.
Figure 3.
The Association between Protein Intake as % of Total Energy Intake (%TEI) and Incident Fragility (Low-Trauma) Fracture and Incident Main Fracture among Men 50+ y and Postmenopausal Women 50+ y
The solid black line is the hazard ratio, while the dotted lines indicate the range of the 95% CI. All models adjusted for age, height, TEI, center, education, smoking, alcohol intake, physical activity, sedentary hours, calcium and vitamin D supplement use, hormone therapy (women only), bisphosphonate use (women only), and diagnosis of osteoporosis (women only).
Discussion
Low protein intakes (<11–12% TEI) in our data were associated with increased risk of fragility fracture and main osteoporotic fracture in post-menopausal women and men ages 50+ y and older compared to moderate intake (15% TEI). Higher protein intakes (15–20% TEI) were associated with similar fracture risk when compared to moderate intake. We stratified by sex based on marked sex differences in risk factors for fracture, but we found that low protein intake was associated with roughly the same increased risk (about double) among both men and women. Our results suggest that the Acceptable Macronutrient Distribution Range of 10–35% protein includes values in the lower end that may be suboptimal, especially for those at high risk of fracture.
Unlike some previous protein intake and fracture studies (11;13), we did not find significant heterogeneity between animal vs. plant sources of protein with respect to fracture outcomes. Given the inconsistent results of other studies, this result is perhaps not unexpected, but given that we observed heterogeneity of BMD by protein source, it needs further explanation. BMD is the most important risk factor for fragility fracture (22), but there are other risk factors for fracture, including those that increase the risk of falling (23). Muscle strength is an important unmeasured risk factor that may play a role in the observed association between protein intake and fracture. It has been suggested that protein intakes above the RDA may lead to improved muscle mass, strength, and function in older adults (24). A study in post-menopausal women found impaired muscle function among women with protein intake below vs above the RDA (25). In the Women’s Health Initiative, postmenopausal women with the highest protein intake had the lowest risk of frailty (a composite endpoint including measures of muscle strength and function) (26). In the Health, Aging, and Body Composition study, community-dwelling men and women with the highest protein intake had the smallest decline in lean mass (27). With regard to protein sources, a recent study in Chinese women found no association between total protein intake and muscle loss, but did find an association between lower protein intake from vegetable sources and increased bone loss (28). Maintenance of muscle function may help preserve BMD, while loss of muscle mass or frailty can predict bone loss (29–31) and fracture (32). In previous work we showed that a nutrient-dense diet was associated with lower fracture risk among post-menopausal women (33). Thus, women consuming high levels of protein from plant sources might also have higher nutrient density leading to lower fracture risk for a given BMD. Finally, the present study may have been underpowered to find modest differences in effect on fracture by protein source.
We found heterogeneity by source in our cross-sectional analysis with BMD as outcome. Plant protein was associated with lower total hip BMD (significant for women) while dairy protein was associated with higher total hip BMD (significant for men and women 50+ y) with similar source specific associations noted at the lumbar spine. The review of Darling et al. considered all cross-sectional studies relating protein intakes to BMD, regardless of whether they considered animal vs. plant protein separately (9). Furthermore, by assessing the relationship both with and without BMI we identified that there are relationships between protein intake and BMD that may be mediated by BMI (i.e. plant-based proteins, non-dairy animal protein), and relationships that are prominent even after adjustment for BMI (i.e. dairy intake). Thus, it is not entirely surprising that studies assessing the association between total protein intake and BMD might yield conflicting results, which may depend in part on the population, the underlying dietary patterns, and the distribution of the protein intake within the three sources of intake.
We found that a higher intake of dairy protein was associated with higher BMD. A recent study has noted that dairy intake is concurrently associated with lower bone turnover markers and higher BMD (34). One possible explanation for this finding is that dairy sources of protein have higher calcium content than non-dairy sources. Furthermore, dairy intake includes milk, which is fortified with vitamin D in Canada. This is supported by our previous findings that milk is associated with higher 25OHD levels in men and women (35) and that total calcium and total vitamin D intake over time are positively associated with BMD (36). Furthermore, the vitamin D content of these sources should be highest in dairy protein, intermediate in non-dairy protein, and lowest in plant protein; thus if vitamin D intake contributes to the higher BMD, then the non-dairy animal protein group should have had an intermediate BMD between the dairy protein and plant protein groups, as observed. A second possibility is that other differences in nutrient content between dairy protein and non-dairy protein sources may have beneficial effects on BMD. A third possibility is that the predominant source of protein in the diet is an indicator of other lifestyle factors that influence BMD, and which were not captured in the adjustment for known confounders.
Our longitudinal analyses showed that the cross-sectional differences observed at Year 5 were only slightly attributable to the change in BMD over the initial five years, and thus the main differences by intake were present at baseline as well. The time sequence means that if the association is causal then it is attributable to long-term average protein intake, which in turn is associated with the protein intake assessed at Year 2. Ideally, we would have had multiple dietary assessments to determine the stability of the total diet and dietary protein over time. We do know, based on an abbreviated FFQ, that dietary calcium intake is relatively stable over time in the CaMos cohort (36). Other studies have shown that dietary patterns remain relatively stable over time (16). Other studies have likewise observed effects in longitudinal analyses that were weaker than what would be predicted by the cross-sectional assessments.
The strengths of this study are the inclusion of major potential confounders and a large population-based sample. We also included both men and women, but did a stratified analyses allowing for sex-differences in risk factors and etiology of fragility fracture. We assessed protein intake by three source categories, differentiating between dairy and non-dairy animal protein (as the associated calcium content might affect bone parameters) and animal vs. plant-based proteins. We noted heterogeneous and opposing relationships, which could lead to relationships that could be in any direction for total protein, depending on the distribution by source. We performed complete case analyses, which in the present study only had a marginal effect on the confidence intervals. Sensitivity analysis for missing exposure data or missing outcomes found that those with missing data were similar to those who had data and were included in the study. The limited scope and specified portion size of the FFQ may yield biased estimates of absolute energy intake and hence the association between total intake and fracture risk might be biased toward the null (37). Under-representation of ethnic minorities in the study may limit generalizability as less than 5% of the study population identified as non-white. The main findings concerning fracture are not generalizable to men and women younger than 50 years. Finally, we cannot rule out the possibility of residual confounding since low protein intake may be related to unmeasured confounders.
In summary, the balance of evidence from this study suggests that adequate protein (> 12% TEI, ideally 14–15% TEI) is an important modifiable risk factor associated with reduced risk of fragility fracture. We found no evidence of a negative effect of higher protein intake on any bone health outcome within the range of usual intakes (roughly 10–20% TEI).
Footnotes
Author Contributions: LL, SIB, CB, NK, DG designed the study. LL performed the data analysis. All authors were involved in drafting and revising and have read and approved the final manuscript. DG has primary responsibility for the final content.
Author Disclosures: Dr. Langsetmo received salary from CaMos; CaMos was funded by grants from Dairy Farmers of Canada, the Canadian Institute for Health Research, Amgen, Merck, and Novartis during the conduct of the study. Dr. Barr reports other activity for Dairy Farmers of Canada outside the submitted work. Ms. Berger received salary from CaMos; CaMos was funded by grants from Dairy Farmers of Canada, the Canadian Institute for Health Research, Amgen, Merck, and Novartis during the conduct of the study. Dr. Adachi has received research grants from Amgen, Eli Lilly and Merck. He serves on advisory boards for Amgen, Eli Lilly and Merck. He is a speaker for Actavis, Amgen, Eli Lilly and Merck. He sits on the Board of Directors and the Scientific Advisory Council for the International Osteoporosis Foundation. He is an advisor for Osteoporosis Canada. Dr. Papaioannou reports grants or research support from Amgen, Eli Lilly, Merck, Warner Chilcott, honoraria/consulting fees from Amgen and Eli Lilly outside the submitted work Dr. Kaiser reports grants from CIHR, grants from Amgen, during the conduct of the study; grants and personal fees from Amgen, grants and personal fees from Eli Lilly, personal fees from Merck, outside the submitted work. Dr. Hanley reports grants and personal fees from Amgen Canada, grants and personal fees from Eli Lilly Canada, grants and personal fees from Novartis, grants from NPS Pharmaceuticals, grants and personal fees from Merck, outside the submitted work. Dr. Kovacs reports personal fees from Amgen, during the conduct of the study. Dr. Josse reports grants from Merck, grants from Eli Lilly, grants from Amgen, outside the submitted work. Dr. Goltzman reports grants from Amgen, grants from Eli Lilly, grants from Dairy Farmers of Canada, grants from Merck, grants from Novartis, during the conduct of the study; personal fees from Amgen, personal fees from Lilly, outside the submitted work. Drs. Prior, Kreiger, and Rahme have no disclosures. The CaMos Research Group: David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto), CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzanne Godmaire (research assistant), Silvia Dumont (administrative assistant), Claudie Berger (study statistician), Lisa Langsetmo (Fellow), CaMos Imaging Centre, Quebec City, Quebec: Jacques P. Brown (director), Brian Lentle (researcher radiologist); Louise Mailloux and Diane Bastien (radiology technologists); Loralee Robertson (radiology technologist for baseline data). Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator). Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator). Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (Coordinator). Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Wilma Hopman (researcher), Karen Rees-Milton (coordinator). University of Toronto, Toronto, Ontario: Robert Josse (director), Sophie Jamal (co-director), Angela M. Cheung (researcher), Barbara Gardner-Bray (coordinator). McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator). University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator). University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator). University British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Millan Patel (co-director), Brian Lentle (researcher/radiologist), Nerkeza Andjelic (coordinator) and Susan I Barr (associated researcher). McGill University, Montreal, Quebec: Elham Rahme (biostatistician), Brent Richards (researcher), Suzanne Morin (researcher). University of Alberta, Edmonton, Alberta: Stuart Jackson (medical physicist). University of Manitoba, Winnipeg, Manitoba: William D. Leslie (researcher/nuclear medicine physician).
Disclosures: This project was supported by a grant from the Canadian Agri-Science Clusters Initiative, Dairy Research Cluster (Dairy Farmers of Canada, Agriculture and Agri-food Canada, Canadian Dairy Commission). CaMos is also funded by: Canadian Institutes of Health Research (CIHR), Amgen, Merck, Eli Lilly, and Novartis. The funding sources were arms-length, and played no role in the data collection, analysis, or interpretation of the results.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine,Panel on Macronutrients and Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington D.C: National Academies Press; 2005. pp. 769–879. [Google Scholar]
- 3.Allen LH, Oddoye EA, Margen S. Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr. 1979;32:741–9. doi: 10.1093/ajcn/32.4.741. [DOI] [PubMed] [Google Scholar]
- 4.Cao JJ, Johnson LK, Hunt JR. A diet high in meat protein and potential renal acid load increases fractional calcium absorption and urinary calcium excretion without affecting markers of bone resorption or formation in postmenopausal women. J Nutr. 2011;141:391–7. doi: 10.3945/jn.110.129361. [DOI] [PubMed] [Google Scholar]
- 5.Fenton TR, Tough SC, Lyon AW, Eliasziw M, Hanley DA. Causal assessment of dietary acid load and bone disease: A systematic review and meta-analysis applying Hill’s epidemiologic criteria for causality. Nutr J. 2011;10:41. doi: 10.1186/1475-2891-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapuri PB, Gallagher JC, Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutr. 2003;77:1517–25. doi: 10.1093/ajcn/77.6.1517. [DOI] [PubMed] [Google Scholar]
- 7.Devine A, Dick IM, Islam AF, Dhaliwal SS, Prince RL. Protein consumption is an important predictor of lower limb bone mass in elderly women. Am J Clin Nutr. 2005;81:1423–8. doi: 10.1093/ajcn/81.6.1423. [DOI] [PubMed] [Google Scholar]
- 8.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly : the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–44. doi: 10.1093/aje/155.7.636. [DOI] [PubMed] [Google Scholar]
- 9.Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90:1674–92. doi: 10.3945/ajcn.2009.27799. [DOI] [PubMed] [Google Scholar]
- 10.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–12. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 11.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol. 1996;143:472–9. doi: 10.1093/oxfordjournals.aje.a008767. [DOI] [PubMed] [Google Scholar]
- 12.Meyer HE, Pedersen JI, Loken EB, Tverdal A. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am J Epidemiol. 1997;145:117–23. doi: 10.1093/oxfordjournals.aje.a009082. [DOI] [PubMed] [Google Scholar]
- 13.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69:147–52. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 14.Sahni S, Cupples LA, McLean RR, et al. Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham offspring cohort. J Bone Miner Res. 2010;25:2494–500. doi: 10.1002/jbmr.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerstetter JE, Kenny AM, Insogna KL. Dietary protein and skeletal health: a review of recent human research. Curr Opin Lipidol. 2011;22:16–20. doi: 10.1097/MOL.0b013e3283419441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 17.Berger C, Langsetmo L, Joseph L, et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Can Med Assoc J. 2008;178:1660–8. doi: 10.1503/cmaj.071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Health Canada. Canadian Nutrient File 2010. Health Canada; 2010. Internet: www.healthcanada.gc.ca/cnf. [Google Scholar]
- 20.Genant HK. Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1995;10:997–8. doi: 10.1002/jbmr.5650100624. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44:734–43. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 22.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19:1431–44. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr. 2012;108(Suppl 2):S88–S93. doi: 10.1017/S0007114512002590. [DOI] [PubMed] [Google Scholar]
- 25.Gregorio L, Brindisi J, Kleppinger A, et al. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J Nutr Health Aging. 2014;18:155–60. doi: 10.1007/s12603-013-0391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beasley JM, Lacroix AZ, Neuhouser ML, et al. Protein intake and incident frailty in the Women’s Health Initiative observational study. J Am Geriatr Soc. 2010;58:1063–71. doi: 10.1111/j.1532-5415.2010.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 28.Chan R, Leung J, Woo J, Kwok T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J Nutr Health Aging. 2014;18:171–7. doi: 10.1007/s12603-013-0379-y. [DOI] [PubMed] [Google Scholar]
- 29.Matsui Y, Takemura M, Harada A, Ando F, Shimokata H. Effects of knee extensor muscle strength on the incidence of osteopenia and osteoporosis after 6 years. J Bone Miner Metab. 2013;32:550–55. doi: 10.1007/s00774-013-0528-8. [DOI] [PubMed] [Google Scholar]
- 30.Sternberg SA, Levin R, Dkaidek S, Edelman S, Resnick T, Menczel J. Frailty and osteoporosis in older women-a prospective study. Osteoporos Int. 2014;25:763–8. doi: 10.1007/s00198-013-2471-x. [DOI] [PubMed] [Google Scholar]
- 31.Go SW, Cha YH, Lee JA, Park HS. Association between sarcopenia, bone density, and health-related quality of life in Korean Men. Korean J Fam Med. 2013;34:281–8. doi: 10.4082/kjfm.2013.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175–80. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Langsetmo L, Hanley DA, Prior JC, et al. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged >/= 50 y: a population-based cohort study. Am J Clin Nutr. 2011;93:192–9. doi: 10.3945/ajcn.110.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunn CA, Weber JL, Kruger MC. Diet, weight, cytokines and bone health in postmenopausal women. J Nutr Health Aging. 2014;18:479–86. doi: 10.1007/s12603-014-0002-x. [DOI] [PubMed] [Google Scholar]
- 35.Berger C, Greene-Finestone L, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-Hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res. 2012;27:1381–9. doi: 10.1002/jbmr.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Langsetmo L, Berger C, et al. Longitudinal changes in calcium and vitamin D intakes and relationship to bone mineral density in a prospective population-based study: the Canadian Multicentre Osteoporosis Study (CaMos) J Musculoskelet Neuronal Interact. 2013;13:470–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Prentice RL, Pettinger M, Tinker LF, et al. Regression calibration in nutritional epidemiology: example of fat density and total energy in relationship to postmenopausal breast cancer. Am J Epidemiol. 2013;178:1663–72. doi: 10.1093/aje/kwt198. [DOI] [PMC free article] [PubMed] [Google Scholar]