Abstract
Previous rodent models of repetitive transcranial magnetic stimulation (rTMS) adopted whole-brain stimulation instead of unilateral hemispheric rTMS, which is unlike the protocols used for human subjects. We report a successful application of rTMS to the unilateral hemisphere of rat brain. The rTMS was delivered with a low-frequency (1 Hz), high-frequency (20 Hz), or sham stimulation protocol to one side of the brain by using a small 25-mm figure-8 coil. We placed the center of the coil 1 cm lateral to the vertex on the biauricular line and angulated the coil 45° to the ground to minimize a potential direct effect of rTMS on the contralateral cortex. We also used an in-house water cooling system to enable repetitive magnetic stimulation for more than 20 min, even at a 20-Hz stimulation frequency. Increases in the transcriptions of immediate early genes (Arc, Junb, and Egr2) were greater after rTMS than after sham stimulation. After 5 consecutive days of 20-min 1-Hz rTMS, bdnf mRNA expression was significantly higher in stimulated cortex than in contralateral side. The model presented herein will elucidate the molecular mechanisms of rTMS by allowing analysis of the inter-hemispheric difference in its effect.
Keywords: Behavior, Issue 116, Transcranial magnetic stimulation, neuronal plasticity, animal models, cerebral cortex, positron emission tomography, immediate early genes, biomedical engineering
Introduction
Repetitive transcranial magnetic stimulation (rTMS), a tool for non-invasive brain stimulation and neuromodulation, has been applied in the treatment of various conditions such as central pain1,2, depression3, migraine4, and even stroke5-7. Rapidly changing electrical current through coils on the head induces an electrical field on the cerebral cortex and a resultant neuronal activation. The excitability of the cerebral cortex can be modulated by rTMS, which can last for more than 30 min after the stimulation is terminated.
Suggested mechanisms of the rTMS after-effect include long-term potentiation/depression-like effect8, transient shift in ionic balance9, and metabolic changes10. In addition, Di Lazzaro et al. suggest that intermittent theta-burst stimulation affects the excitatory synaptic inputs to pyramidal tract neurons, both in the stimulated and the contralateral hemisphere11.
Significant limitations, however, have hindered researchers from translating on-bench evidence to clinical situations. First, in previous animal studies, rTMS was used for whole-brain stimulation12. Whole-brain stimulation is quite different from the protocols used in human studies9. The other problem is related with the stimulation duration. This is at least partly attributable to the fact that an effective cooling system was unavailable for small coils in the past.
In recent years, seminal articles have been published suggesting ways for overcoming these difficulties in the rTMS experiment on the small animal brain. By these animal models, it was revealed that the rat brain also shows similar cortical excitability changes as in human in response to low-frequency rTMS13. More importantly, cellular and molecular mechanisms of rTMS are increasingly being investigated using animal models of rTMS. A case in point is that a distinct type of inhibitory interneuron is known to be most sensitive to intermittent theta burst stimulation14. Rodent models of rTMS, thus, offer new opportunities for exploring much-sought questions on the molecular underpinnings of rTMS-induced changes. If small animal models of rTMS can be used in more laboratories, it may greatly accelerate and strengthen research in this area.
We now describe how to apply rTMS to the unilateral hemisphere of rat brain, an extension of the previous work15. Stimulation-induced changes were evaluated by using micro-positron emission tomography (PET) and mRNA microarrays to study rTMS-induced changes in the stimulated cerebral cortex.
Protocol
All of the procedures using animals were reviewed and approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital.
1. Experimental Setup
- Animal preparation
- Allow male Sprague-Dawley rats 1 week to adapt to their new environment before starting the experiment. NOTE: Although 8-week old rats were used in the present study, a developing or adult brain can be chosen according to the research hypotheses.
- Inhalation anesthesia for induction
- Induce and maintain anesthesia with 5% and 2% of isoflurane dissolved in 40%/60% and 25%/75% oxygen/nitrogen via a chamber and nose cone, respectively. Adjust anesthesia depth to the level of abolishing the pedal withdrawal reflex to toe pinch to confirm proper anesthetization. NOTE: Using awoken animals can be a better choice in translational terms, but there are difficulty to restrain during rTMS and they are prone to excessive stress.
- Monitor body temperature with a rectal probe and maintain it at 37 °C by using a homeothermic blanket. Monitor anesthetic depth using pedal withdrawal reflex, temperature, respiratory rate and heart rate.
- Switch-over to intravenous anesthesia for maintenance
- Prepare the tail with an alcohol swab. Catheterize a lateral tail vein with a 24-gauge venous catheter for transition to IV anesthesia (Figure 1A). Load propofol intravenously (1 mg/[kg·min] over 10 min, using 10 mg/ml emulsion) to the animals. Discontinue isoflurane 5 min after starting propofol loading.
- Maintain propofol sedation at an infusion rate of 500 - 700 µg/(kg·min) throughout the experiment, as in a previous study16. Supplement oxygen at 0.8 L/min via a nose cone. NOTE: Anesthesia with propofol is to reduce potential suppression of cortical excitability by the inhalation agent17-19. However, anesthesia is not mandatory in rTMS experiments, and awoken animals can also be used. Anesthesia methods should be decided in consideration of the research hypotheses.
- Use veterinary ointment on eyes to prevent dryness while under anesthesia.
- Apply magnetic stimulation (see Section2) 10 min after complete transition to i.v. anesthesia.
- Recovery conditions
- Monitor vital signs during recovery phase. Do not leave the animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. If an animal has undergone surgery, do not return it to the company of other animals until fully recovered. NOTE: If surgery for a disease model is performed, post-surgical pain management is necessary. However, pain management is not needed for this rTMS experiment.
2. Repetitive Transcranial Magnetic Stimulation
- Stimulator and coil
- Apply stimulation by using a repetitive stimulator that delivers biphasic stimuli via a 25 mm figure-8 coil. Locate the center of the coil 0.5 cm lateral to the vertex on the biauricular line, and angulate the coil 45° to the ground. NOTE: The maximal magnetic field strength of the coil is 4.0 T. The magnetic coil is mounted firmly on a built-in holder.
- Motor threshold
- Determine the motor threshold (MT) at the hot spot, with the center of the coil positioned 0.5 cm lateral to the vertex on the biauricular line and with the surface flat onto the calvaria. This is the same methodology used in a previous study20. NOTE: Define MT as the minimum stimulus intensity evoking 5 or more palpable contractions on the contralateral forepaw by 10 consecutive stimuli. Verify whether the stimulation is primarily causing contralateral muscle contraction to ensure unilateral stimulation.
- Application of rTMS
- Apply rTMS 10 min after stabilization of deep anesthesia. Place the center of the coil at the target rTMS site, selected from the cerebral cortices depending on research questions. Then, tilt the coil to ensure direct contact between the coil center and the surface of skull at the stimulation point. NOTE: For example, angulate the coil 45° to the ground to minimize a potential direct effect of rTMS on the contralateral cortex (Figure 1B and 1C).
- Subject the animals to a session of 20-min rTMS of the unilateral hemisphere. Using the software console, deliver rTMS with a low-frequency (1 Hz), high-frequency (20 Hz), or sham stimulation protocol, and set the stimulation intensity at 100 - 110% of the MT.
- Perform 1 Hz stimulation without rest. Using the software console input "1,200" shots for "20" min). For 20 Hz stimulation, conduct 2 sec of stimulation followed by 28 sec of rest. Using the software console input "1,600" shots for "20" min.
- For sham stimulation, tilt the coil perpendicular (90° rotation) to the calvaria and place the coil edge 2 cm apart from head surface (Figure 1D). Fix the coil holder firmly to the main apparatus; there is no need to hold the coil by hand during the experiment. NOTE: To compensate for acoustic and other non-specific effects, distinct sham protocols should be used for distinct stimulation protocols. For example, 1-Hz sham stimulation can be used for 1-Hz rTMS experiments.
- Cooling the coil
- Use a water cooling system to enable repetitive magnetic stimulation for more than 20 min at 1- and 20-Hz stimulation frequencies (Figure 2). Circulate icy water surrounding the whole length of the coil during the experiment to prevent overheating, although the temperature of the coil or stimulator is not monitored. NOTE: Commercially available cooled rat coils can also be used.
- If possible, monitor the coil temperature by viewing the heating gauge of the rTMS machine. NOTE: There were no adverse consequences related to the rTMS stimulation. There is, however, a potential burn risk if metal ear identification tags are used near the stimulating coil.
3. Micro Positron Emission Tomography
- Animal preparation
- Conduct inhalation anesthesia for induction and i.v. anesthesia for maintenance (see step 1.2.1 and 1.3.1). Apply 1-Hz rTMS to an animal for 10 min at a stimulation intensity of 100-110% of the MT.
- Five minutes after finishing the rTMS stimulation, inject 1 mCi of 2-[F-18]fluoro-deoxyglucose (18FDG) dissolved in 0.5 ml of normal saline intravenously by using a tail vein catheter. Allow 30 min for 18FDG uptake. NOTE: Place the rat under anesthesia during the entire micro-PET experiment.
- Image analysis
- Use a PET scanner for brain imaging to reaffirm the unilaterality of the stimulation. Reconstruct images with a 3-D iterative algorithm. To assess the changes in metabolism induced by rTMS, identify regions of interest (ROIs) in the images of the transverse brain sections21.
- Euthanasia
- After performing micro-PET imaging, euthanize the rats in a chamber prefilled with carbon dioxide while the rats are in deep anesthesia.
4. mRNA Microarray
- Euthanasia
- Induce and maintain anesthesia with 5% and 2% of isoflurane dissolved in 40%/60% and 25%/75% oxygen/nitrogen via a chamber and nose cone, respectively. Anesthetize deeply to the level of abolishing the pedal withdrawal reflex to toe pinch before being decapitated.
- Decapitate the rats for euthanasia 5 min after 1 session of 1-Hz rTMS.
- Tissue harvest
- Lay out materials and surgical instruments in the order of use, including folded paper towels, a bone rongeur, microscissors, larger surgical scissors, a microforcep, a No. 10 or 11 scalpel blade, a lidded 10-cm glass petri dish filled with ice, and 1.5-ml tubes. Prepare a plastic bag for disposal of carcass.
- Make a midline skin incision in the skull anterioposteriorly. Bluntly dissect the soft tissue and surrounding muscles with surgical scissors, and remove the skull bone piece using a bone rongeur. Quickly dissect the fresh brain carefully from the skull. Then, lay it on ice by using the microforceps and microscissors. Rinse the brain tissue in ice-cold normal saline.
- Transfer the brain to dry ice immediately, and subsequently store it at -80 °C in a tube until further processing.
- Thaw the brain tissue before harvest.
- Place the brain dorsal side up, and harvest the brain tissue from the stimulated cerebral cortex (around the hot spot in the primary motor cortex) on ice by using the microforceps and microscissors. Put the harvested tissue in the 1.5-ml tube.
- RNA preparation
- Extract total RNA from tissue homogenates using lysis reagent22. Process with DNase digestion and clean-up procedures. Quantify the RNA samples and aliquots and store them at -80 °C until use.
- For quality control, evaluate RNA purity and integrity by denaturing gel electrophoresis, at a OD ratio of 260:280, and analyze them on a commercially available analyzer.
- Labeling and purification
- Amplify and purify total RNA by using a commercially available RNA amplification kit to yield biotinylated cRNA. Briefly, reverse-transcribe 550 ng of total RNA to cDNA by using a T7 oligo(dT) primer. Synthesize and in vitro transcribe the second-strand cDNA and then label it with biotin-NTP.
- After purification, quantify cDNA by using a spectrophotometer.
- Hybridization and data export23
- Use the expression beadchip for mRNA expression analysis. Hybridize the labeled 750-ng cDNA samples to each rat-12 expression bead array for 16 - 18 hr at 58 °C. Carry out detection of array signal by using streptavidin-Cy3.
- Scan arrays with a confocal scanner. Perform array data export processing and analysis by using a commercially available software.
Representative Results
Fifteen 8-week old male Sprague-Dawley rats were used for a separate inter-rater reliability analysis of MT determination. Using palpation of muscle twitching, the MTs were obtainable in all rats and measured as 33.00 ± 4.21% maximal stimulator output (% MSO) and 33.93 ± 0.88% MSO, respectively, by two independent researchers. Bland-Altman bias was -0.93, and the 95% limits of agreement were -9.13 to 7.26%.
In the micro-PET experiment on six 8-week old rats (n= 4 in the 1-Hz rTMS, and n= 2 in the sham rTMS group), the uptake of 18F-FDG in the ROIs was calculated as the averaged nCi/cc after calibration of both ipsilateral and contralateral cerebral cortices in the same images. The radioactivity in the contralateral area was used as a reference to normalize data obtained in the ipsilateral area, and the differential uptake ratio (DUR) was calculated. The mean DURs obtained from three consecutive transverse images were averaged to obtain the DURs for the rats. This is the same methodology used in a previous study21. 18FDG-PET images showed a focal increase in glucose metabolism in the stimulated left cortical area in the 1-Hz group, supporting the unilaterality of the rTMS (Figure 3).
In the mRNA microarray study, the quality of hybridization and overall chip performance were monitored by visual inspection of both the internal quality control checks and raw scanned data. Array data were filtered according to a detection p value of < 0.05 (similar to the signal-to-noise ratio) in at least 50% samples (a higher signal value was required to obtain a detection p value of < 0.05). The selected gene signal value was transformed by logarithm and normalized by using a quantile method. The statistical significance of the expression data was determined by using the Mann-Whitney U test and fold change, in which the null hypothesis was that no difference exists between the 1-Hz rTMS (n = 4) and sham groups (n = 4). The false discovery rate was controlled by adjusting the p value by using the Benjamini-Hochberg algorithm. After normalization and filtering, mRNAs showing significant differential expressions (|fold change| 1.2, p < 0.05) were selected. As a result, the expression levels of the immediate early genes were significantly higher in the rTMS group than in the sham group, with the expressions of the Arc, Junb, and Egr2 genes upregulated (Figure 4A).
In addition, we measured bdnf mRNA expressions in the stimulated and contralateral cortex after 5 consecutive days of 20-min rTMS (n = 5 each in the 1-Hz and 20-Hz groups). After 1-Hz stimulation, bdnf mRNA expression was significantly higher in the stimulated cortex than in the contralateral one (Figure 4B). This revealed differential rTMS-induced changes in the stimulated and contralateral cerebral cortices.
 Figure 1.Experimental Settings.(A) An intravenous catheter is inserted at a lateral tail vein (arrow), and a nose cone is used for anesthesia with isoflurane as well as for oxygen supplement after a switch-over to intravenous propofol. (B) Dorsal anterolateral view during rTMS. (C) Dorsal posterior view. The surface of a figure-of-8 coil is angulated 45° to the ground to minimize the potential direct stimulation of the contralateral cortex. (D) A schematic illustration of sham rTMS. The coil is placed 2 cm away from and tilted perpendicular (90° rotation) to the calvaria. Please click here to view a larger version of this figure.
Figure 1.Experimental Settings.(A) An intravenous catheter is inserted at a lateral tail vein (arrow), and a nose cone is used for anesthesia with isoflurane as well as for oxygen supplement after a switch-over to intravenous propofol. (B) Dorsal anterolateral view during rTMS. (C) Dorsal posterior view. The surface of a figure-of-8 coil is angulated 45° to the ground to minimize the potential direct stimulation of the contralateral cortex. (D) A schematic illustration of sham rTMS. The coil is placed 2 cm away from and tilted perpendicular (90° rotation) to the calvaria. Please click here to view a larger version of this figure.
 Figure 2.The Cooling System uses a Water-circulating Pump with Motor. Ice packing on the copper wires of the coil is not needed, as the cooling system enwrapping the cable of the coil is sufficient to cool the heat produced at the copper wires. The surface of the coil is not in direct contact with the iced water. The cooling system is active during stimulation sessions.
Figure 2.The Cooling System uses a Water-circulating Pump with Motor. Ice packing on the copper wires of the coil is not needed, as the cooling system enwrapping the cable of the coil is sufficient to cool the heat produced at the copper wires. The surface of the coil is not in direct contact with the iced water. The cooling system is active during stimulation sessions.
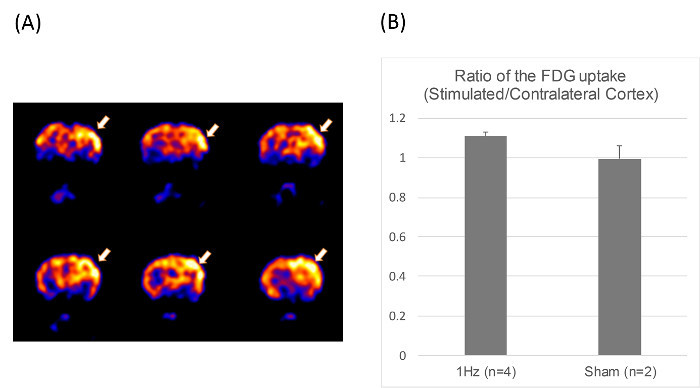 Figure 3.Positron Emission Tomography (PET) Image.(A) The coronal sections of micro-PET images of a rat obtained using 2-[F-18]fluoro-deoxyglucose, showing increased local glucose metabolism in the stimulated cortex after 1-Hz rTMS for 10 min at 100% of the MT (arrows). (B) The ratio of FDG uptake in stimulated/contralateral cortex in the 1-Hz (n= 4) and sham rTMS group (n= 2). Please click here to view a larger version of this figure.
Figure 3.Positron Emission Tomography (PET) Image.(A) The coronal sections of micro-PET images of a rat obtained using 2-[F-18]fluoro-deoxyglucose, showing increased local glucose metabolism in the stimulated cortex after 1-Hz rTMS for 10 min at 100% of the MT (arrows). (B) The ratio of FDG uptake in stimulated/contralateral cortex in the 1-Hz (n= 4) and sham rTMS group (n= 2). Please click here to view a larger version of this figure.
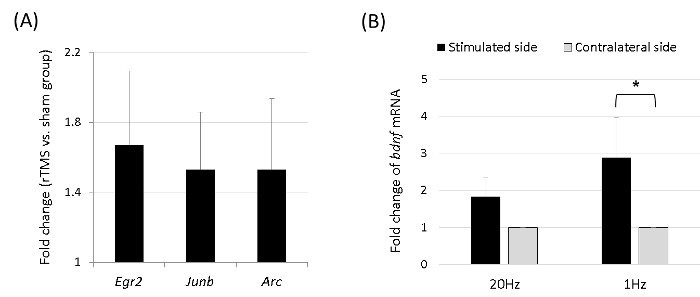 Figure 4.The mRNA Microarray of the Immediate Early Genes and bdnf.(A) Arc, Junb, and Egr2 were differentially expressed, which were identified on the microarray obtained 5 min after 1 session of 1-Hz rTMS, ordered by fold change. The expression levels of the genes were significantly higher in the rTMS group (n = 4) than in the sham group (n = 4) (p < 0.05 with Mann-Whitney U test), with the expressions of the Arc, Junb, and Egr2 genes upregulated. (B) After 5 consecutive days of 20-min 1-Hz rTMS, bdnf mRNA expression was significantly higher in the stimulated cortex than in the contralateral side (*p < 0.05, Wilcoxon signed-rank test). Please click here to view a larger version of this figure.
Figure 4.The mRNA Microarray of the Immediate Early Genes and bdnf.(A) Arc, Junb, and Egr2 were differentially expressed, which were identified on the microarray obtained 5 min after 1 session of 1-Hz rTMS, ordered by fold change. The expression levels of the genes were significantly higher in the rTMS group (n = 4) than in the sham group (n = 4) (p < 0.05 with Mann-Whitney U test), with the expressions of the Arc, Junb, and Egr2 genes upregulated. (B) After 5 consecutive days of 20-min 1-Hz rTMS, bdnf mRNA expression was significantly higher in the stimulated cortex than in the contralateral side (*p < 0.05, Wilcoxon signed-rank test). Please click here to view a larger version of this figure.
Discussion
The primary purpose of this study was to introduce an animal model of unilateral rTMS. Although unilateral stimulation is one of the most fundamental characteristics of human rTMS research, many studies have not adopted it in small animals. However, Rotenberg et al.15 recorded contralateral MEPs with stimulation of 100% MT using a figure-8 coil with an outside lobe diameter of 20 mm, whereas stimulation with 112.5% and 133.3% MT produced ipsilateral as well as contralateral MEPs. This might be because the large induced electric field can affect the contralateral hemisphere. Thus, our study is an extension of this previous work15,24, by moving the coil more lateral and tilting it to accentuate unilateral stimulation. The primary aim of this study was achieved because we confirmed that micro-PET revealed a local increase in the glucose metabolism in the stimulated cerebral cortex after rTMS (Figure 3).
Location and angulation of the coil are critical steps in this experiment. Unilateral stimulation is possible by placing the center of the rTMS coil 1 cm lateral to the vertex on the biauricular line and angulating the coil 45° to the ground. The stimulation site can be different from the primary motor cortex (M1), depending on the condition that investigators want to target with rTMS. For instance, to ameliorate depression, the dorsolateral prefrontal cortex (DLPFC) is stimulated with rTMS, but the motor threshold, which is also measured in M1, determines the stimulation intensity even for DLPFC rTMS. Likewise, the hotspot — 0.5 cm lateral to the vertex on the biauricular line — was used to determine the motor threshold in the present study. The more lateral cortex — 1 cm lateral to the vertex — was intentionally selected to ensure the unilaterality of stimulation and investigate rTMS-induced molecular changes.
As for the magnetic field strength within the tissue, in a previous finite element modeling study on the induced electrical field in the mouse brain, the induced electrical field by the 70 mm figure-8 coil at 75% MSO reached approximately 150 V/m on the brain surface and in the cortex. The electric field strength dropped dramatically as distance increased, showing the maximum depth with greater than 100 V/m strength was just 1.9 mm for the 70 mm figure-8 coil25. In another rat study, at 10 mm depth the induced electrical field strength decreased to 25% of that on the brain surface26. Interestingly, the half power region (HPR) was as broad as ~7 x 7 mm (0.51 cm2) even when a 25 mm figure-8 coil was used25. Although concrete numbers were not provided for the 70 mm figure-8 coil, Salvador and Miranda commented that the HPR for the 70 mm coil was larger than that of the 25 mm coil. Since we wanted to prevent the HPR from covering the contralateral hemisphere, we selected a spot 1 cm lateral to the midline. Tilting was inevitable to ensure direct contact between the coil center and the surface of the skull at the stimulation point.
Anesthesia can potentially depress neuronal excitability, glucose metabolism, and gene expression. Haghighi et al. revealed that isoflurane at concentration of 0.5% significantly depressed electrical transcranial MEPs recorded from rats17. On the other hand, MEPs were preserved during propofol infusion as high as 40 mg/[kg·hr], with amplitudes remaining large in rats18. In a human study, no compound muscle action potentials (CMAP) were detected during isoflurane anesthesia. However, 333 Hz, four-pulse magnetic stimulation evoked CMAP in the hypothenar muscle in 75% of patients, and in the anterior tibial muscle in 65% of patients, during propofol anesthesia19. Using awoken animals can be a better choice in the physiological aspects, but they are not easy to restrain during rTMS and are prone to stressful conditions.
As troubleshooting, a simple cooler that used a water-circulating pump enabled us to extend the stimulation duration for more than 20 min even at a 20-Hz stimulation frequency. This is important because it enables as many stimulations as in rTMS protocols for human subjects. Cooling the figure-8 coil with only a handheld ice-cold water bag was not sufficient to ensure stimulation of more than 20 min. Long rTMS duration in small animals will provide the opportunity for in-depth investigation of the molecular mechanisms of rTMS. Commercially available cooled rat coils will be reasonable alternatives.
There were several limitations in this experiment. First, only a biphasic pulse was available, which was a limitation of the rTMS machine we used. Future studies investigating the effect of various pulses and waveforms will be needed. Second, we adopted a pragmatic approach to determine the motor threshold by palpation. Although this method may be inferior to EMG techniques in terms of accuracy, it is readily reproducible and applicable to many research hypotheses. For example, if the primary purpose of a researcher were to investigate differences between the primary motor cortex and adjacent subcortices in rTMS-induced gene or protein expression, more precise determination of motor threshold would be necessary. If a researcher, however, wanted to analyze rTMS-induced gene expression profiles in the dorsolateral prefrontal cortical tissue, the present pragmatic approach may suffice, because the distance and angle between the target tissue and the coil can vary slightly during the movement of the coil from the M1 to the DLPFC area. Third, although we successfully applied rTMS on the unilateral hemisphere of the rat brain, still the stimulation is not as focal as rTMS in human research. The induced strong electric field of ~ 0.5 cm2 on less than 10 cm2 of the rat brain surface seems relatively more diffuse than that in the human hemispheric surface of ~ 2,500 cm2 27. We believe, however, that the model presented herein can be used for elucidating the molecular mechanisms of rTMS by allowing analysis of the inter-hemispheric difference in its effect.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00458). The authors thank Jin-Joo Lee for the technical assistance.
References
- Lefaucheur JP, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75(4):612–616. doi: 10.1136/jnnp.2003.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama A, et al. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006;122(1-2):22–27. doi: 10.1016/j.pain.2005.12.001. [DOI] [PubMed] [Google Scholar]
- O'Reardon JP, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Brighina F, et al. Facilitatory effects of 1 Hz rTMS in motor cortex of patients affected by migraine with aura. Exp Brain Res. 2005;161(1):34–38. doi: 10.1007/s00221-004-2042-7. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS) Neurophysiol Clin. 2006;36(3):105–115. doi: 10.1016/j.neucli.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Fregni F, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation) Nat Rev Neurosci. 2007;8(7):559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176(4):603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586(16):3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A, Keck ME. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms. J Psychiatr Res. 2001;35(4):193–215. doi: 10.1016/s0022-3956(01)00023-1. [DOI] [PubMed] [Google Scholar]
- Muller PA, Dhamne SC, Vahabzadeh-Hagh AM, Pascual-Leone A, Jensen FE, Rotenberg A. Suppression of motor cortical excitability in anesthetized rats by low frequency repetitive transcranial magnetic stimulation. PLoS One. 2014;9(3):91065. doi: 10.1371/journal.pone.0091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke K, Benali A. Modulation of cortical inhibition by rTMS - findings obtained from animal models. J Physiol. 2011;589(18):4423–4435. doi: 10.1113/jphysiol.2011.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, et al. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol. 2010;121(1):104–108. doi: 10.1016/j.clinph.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beom J, Kim W, Han TR, Seo KS, Oh BM. Concurrent use of granulocyte-colony stimulating factor with repetitive transcranial magnetic stimulation did not enhance recovery of function in the early subacute stroke in rats. Neurol Sci. 2015;36(5):771–777. doi: 10.1007/s10072-014-2046-4. [DOI] [PubMed] [Google Scholar]
- Haghighi SS, Green KD, Oro JJ, Drake RK, Kracke GR. Depressive effect of isoflurane anesthesia on motor evoked potentials. Neurosurgery. 1990;26:993–997. doi: 10.1097/00006123-199006000-00012. [DOI] [PubMed] [Google Scholar]
- Fishback AS, Shields CB, Linden RD, Zhang YP, Burke D. The effects of propofol on rat transcranial magnetic motor evoked potentials. Neurosurgery. 1995;37(5):969–974. doi: 10.1227/00006123-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Rohde V, Krombach GA, Baumert JH, Kreitschmann-Andermahr I, Weinzierl M, Gilsbach JM. Measurement of motor evoked potentials following repetitive magnetic motor cortex stimulation during isoflurane or propofol anaesthesia. Br J Anaesth. 2003;91(4):487–492. doi: 10.1093/bja/aeg224. [DOI] [PubMed] [Google Scholar]
- Lee SA, Oh BM, Kim SJ, Paik NJ. The molecular evidence of neural plasticity induced by cerebellar repetitive transcranial magnetic stimulation in the rat brain: a preliminary report. Neurosci Lett. 2014;575:47–52. doi: 10.1016/j.neulet.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Fu YK, et al. Imaging of regional metabolic activity by (18)F-FDG/PET in rats with transient cerebral ischemia. Appl Radiat Isot. 2009;67(18):1743–1747. doi: 10.1016/j.apradiso.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. Am J Physiol Endocrinol Metab. 2007;292(3):820–828. doi: 10.1152/ajpendo.00467.2006. [DOI] [PubMed] [Google Scholar]
- Zidek N, Hellmann J, Kramer PJ, Hewitt PG. Acute hepatotoxicity: a predictive model based on focused illumina microarrays. Toxicol Sci. 2007;99(1):289–302. doi: 10.1093/toxsci/kfm131. [DOI] [PubMed] [Google Scholar]
- Hsieh TH, Dhamne SC, Chen JJ, Pascual-Leone A, Jensen FE, Rotenberg A. A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats. J Neurophysiol. 2012;107(3):966–972. doi: 10.1152/jn.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Miranda PC. Transcranial magnetic stimulation of small animals: a modeling study of the influence of coil geometry, size and orientation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:674–677. doi: 10.1109/IEMBS.2009.5334070. [DOI] [PubMed] [Google Scholar]
- Parthoens J, Verhaeghe J, Servaes S, Miranda A, Stroobants S, Staelens S. Performance Characterization of an Actively Cooled Repetitive Transcranial Magnetic Stimulation Coil for the Rat. Neuromodulation. 2016. [DOI] [PubMed]
- Toro R, et al. Brain size and folding of the human cerebral cortex. Cereb Cortex. 2008;18(10):2352–2357. doi: 10.1093/cercor/bhm261. [DOI] [PubMed] [Google Scholar]


