Scheme 7. Synthesis of Compounds 16–19a.
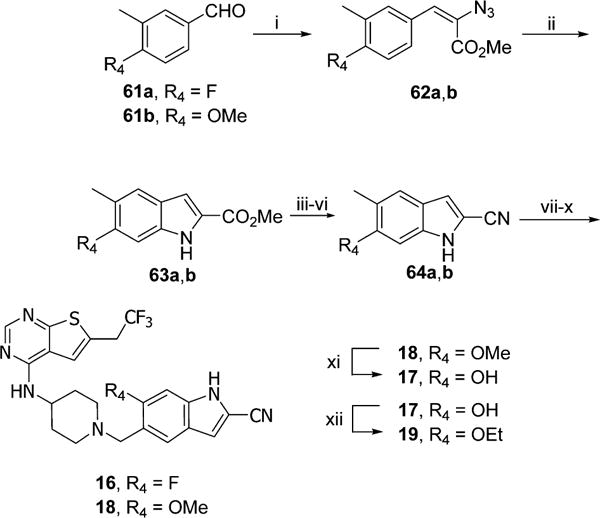
aReagents and conditions: (i) methyl azidoacetate, MeONa, MeOH, −15 to 4 °C, 60–65%; (ii) Rh2(CF3CO2)4, toluene, 50 °C, 24 h, 33–75%; (iii) KOH, MeOH, reflux, 3 h; (iv) oxalyl chloride, DCM, rt, 1 h; (v) NH3·H2O, rt, 2 h; (vi) POCl3, CHCl3, reflux, 4 h, 58–62% (4 steps); (vii) Boc2O, CH3CN, rt, 1 h; (viii) NBS, AIBN, CCl4, reflux, 2 h; (ix) 39, DIEA, DCM, 5 h; (x) SnCl4, CH3CN, 0 °C to rt, 0.5 h, 50–77% (4 steps); (xi) BBr3, DCM, rt, 24 h, 56%; (xii) EtI, K2CO3, CH3CN, rt, 24 h, 61%.
