Scheme 8. Synthesis of Compounds 23, 24, 27–31, 33, and 34a.
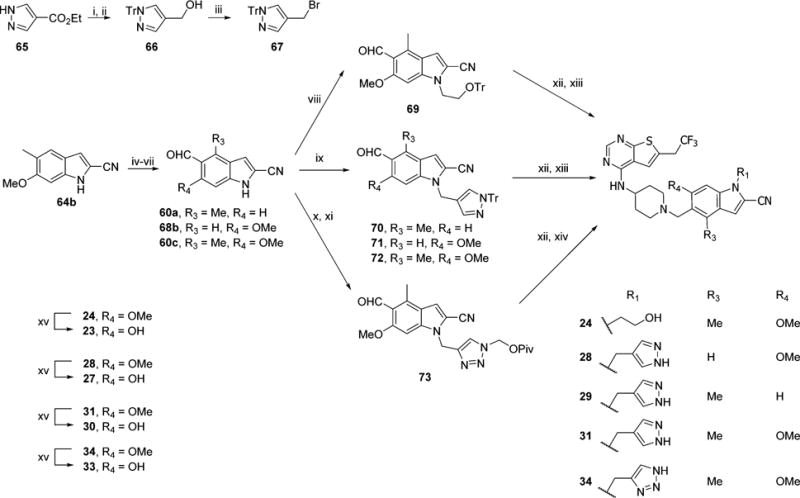
aReagents and conditions: (i) t-BuOK, TrCl, DMF, rt, 2 h; (ii) LAH, THF, 0 °C to rt, 2 h, 97% (2 steps); (iii) NBS, PPh3, DCM, rt, 0.5 h, 79%; (iv) Boc2O, DMAP, CH3CN, rt, 1 h; (v) 2.2 equiv of NBS, AIBN, CCl4, reflux, 5 h; (vi) Ag2CO3, Me2CO/H2O, rt, 24 h (2 steps); (vii) TFA, DCM, rt, 1 h, 38% (4 steps); (viii) BrCH2CH2OTr, Cs2CO3, DMF, rt, 2 h, 50%; (ix) 67, Cs2CO3, DMF, rt, 0.5 h, 82–94%; (x) propargyl bromide, Cs2CO3, DMF, rt, 4 h; (xi) t-BuCO2CH2N3, CuSO4, sodium ascorbate, t-BuOH/H2O, 60 °C, 4 h, 90%; (xii) 39, Et3N, NaBH(OAc)3, DCM, rt, 15 h; (xiii) HCl, MeOH, rt, 1 h, 50–72% (2 steps); (xiv) KOH, MeOH, rt, 1 h, 87% (2 steps); (xv) BBr3, DCM, rt, 24 h, 17–49%.
