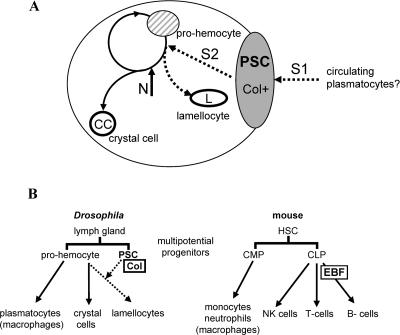
Figure 6. A Model for Lamellocyte Specification.
(A) A model for the induction of lamellocyte differentiation in the Drosophila lymph glands in response to wasp parasitization. Col enables PSC cells to respond to a primary signal (S1) that is likely emitted by plasmatocytes upon their encounter with a parasite (Russo et al. 1996; Meister 2004). As a result, the PSC cells send a secondary signal (S2) that causes prohemocytes to develop into lamellocytes. Notch (N) signalling instructs a fraction of prohemocytes to become crystal cells (Duvic et al. 2002; Lebestky et al. 2003). The circular arrow indicates that increased proliferation leading to increased numbers of crystal cells and lamellocytes follows parasitization (Sorrentino et al. 2002).
(B) Schematic view of hematopoiesis in Drosophila and mouse. Left: Lymph gland cells contain two types of hematopoietic cells, PSC cells and uncommitted precursors. These precursors can give rise to either plasmatocytes or crystal cells. Crystal cell precursors can also give rise to lamellocytes upon receiving a signal from the PSC cells expressing Col (dotted arrows); this signalling is itself dependent upon a communication between circulating plasmatocytes and the PSC (A). Right: In mice, hematopoietic stem cells (HSC) give rise to common myeloid precursors (CMP) and common lymphoid precursors (CLP) (adapted from Orkin [2000] and Schebesta et al. [2002]). Signalling between CMP- and CLP-derived cells is an essential component of adaptive immunity. Col and EBF functions, in Drosophila and vertebrate hematopoiesis, respectively, suggest an ancestral role in their conferring on a subset of hematopoietic cells the ability to respond to signals from circulating immune supervisors (generically designated here as macrophages) and to provide a secondary line of defence against specific intruders.