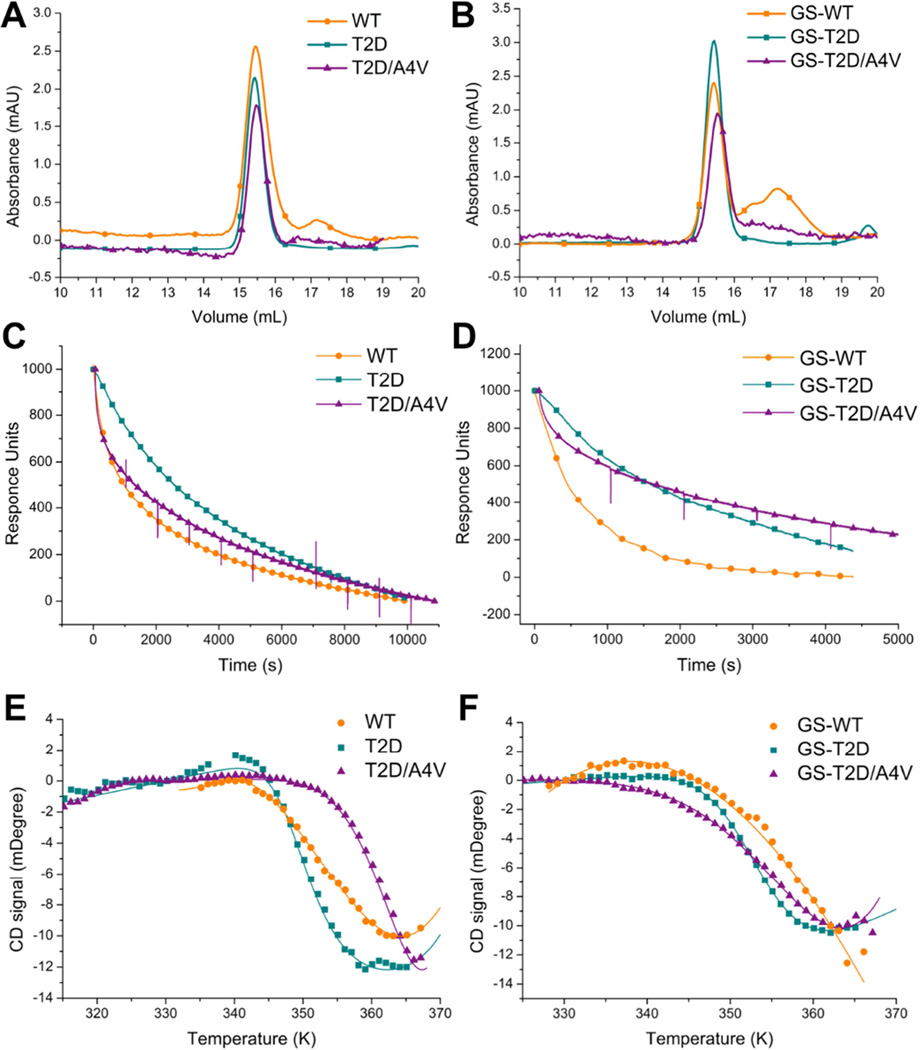
Figure 2. T2D stabilizes the native state of SOD1 through decreased dimer dissociation rates.
(A) Size exclusion chromatograms showing the populations of native dimer (15.5 ml) and monomer (17.4 ml) for unmodified T2D-, T2D/A4V- and WT-SOD1. Samples were taken after incubation at physiological conditions (30 µM SOD1, pH 7.4) and 37°C for 7 days. (B) Size exclusion chromatograms for glutathionylated T2D-, T2D/A4V- and WT-SOD1. Dissociation of immobilized dimers was monitored by surface plasmon resonance for T2D-, T2D/A4V- and WT-SOD1 (C) and glutathionylated species (D). Thermal denaturation curves of unmodified (E) and glutathionylated(F) SOD1 proteins indicate the Tm of T2D-SOD1 is lower than that of WT-SOD1 in the unfolding of SOD1 monomers. Fitting to a two-state model is represented as lines. Size exclusion chromatograms, dissociation profile and thermal denaturation curves of A4V–SOD1 and GS-A4V–SOD1 have been reported (Redler et al., 2011). See also Figure S4.