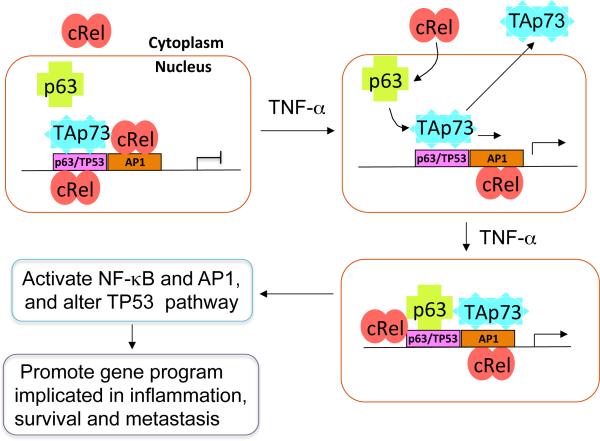
Figure 8. Mechanistic model illustrating effects of TNF-α modulation of cREL, ΔNp63α and TAp73 chromatin occupancy and reprogramming of TAp73 from TP53 to AP-1 sites to promote inflammatory and cancer gene programs.
Most head and neck and solid cancers have high frequencies of TP53 mutation, where TAp73 can serve as a potential tumor suppressor. In this model, without TNF-α, TAp73 predominantly occupies TP53 or p63 binding sites, while NF-κB family member cREL either resides in the cytoplasm or in the nucleus with binding on AP-1 sites, and ΔNp63α is found in the nucleus unbound to DNA (upper left). TNF-α, a major inflammatory cytokine produced in the tumor microenvironment, can promote cREL nuclear translocation, and complexes with ΔNp63α, to occupy TP53/p63 binding sites. TNF-α also induces nuclear displacement or reprogramming of TAp73 to bind neighboring AP-1 sites (upper and bottom right). These dynamic alterations diminish TAp73 tumor suppressor activity, while enhancing cREL/ΔNp63α and TAp73 mediated inflammatory and cancer gene programs (lower left). This model helps explains how TNF-α modulates NF-κB and AP-1 signaling while altering tumor suppressor activity, to promote gene programs implicated in inflammation, survival, and metastasis.