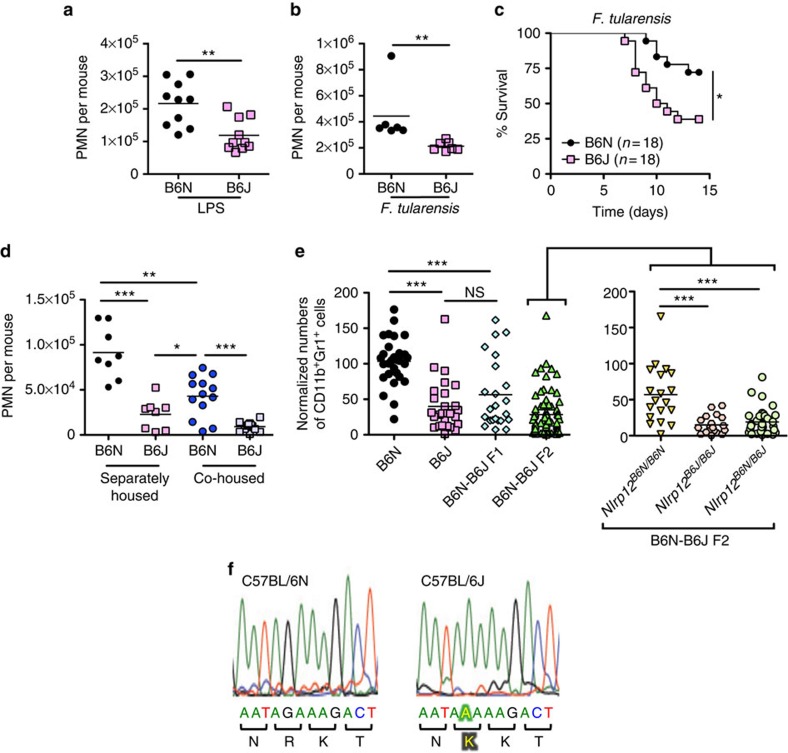
Figure 1. Nlrp12 mutation in C57BL/6J mice results in defective neutrophil recruitment.
(a,b) C57BL/6N (B6N) and C57BL/6J (B6J) mice were challenged i.n. with LPS (0.5 mg kg−1 of body weight) (n=10 per group) (a), or 5 × 103 colony-forming unit (CFU) of F. tularensis LVS (n=6 per group) (b). Six hours (a) or 72 h (b) post challenge BAL were performed and the number of Gr1+ neutrophils quantified by flow cytometry. (c) B6N and B6J mice were challenged i.n. with 5 × 103 CFU of F. tularensis LVS and survival monitored. (d) B6N and B6J mice were either housed separately or co-housed at a 1:1 ratio for 4 weeks before i.n. challenge with LPS. Neutrophil influx (CD45.2+ CD11b+ Gr1+) into the BAL was determined 6 h post challenge by flow cytometry (n=8, separately housed group; n≥12, co-housed group). (e) B6N, B6J, B6N-B6J F1 and B6N-B6J F2 mice were challenged i.n. with LPS. Six hours post challenge BAL were performed and the number of neutrophils (CD45.2+ CD11b+ Gr1+) quantified by flow cytometry. The Nlrp12 allele was sequenced for all B6N-B6J F2 mice and cohorts stratified based on their Nlrp12 genotype (Nlrp12B6N/B6N, Nlrp12B6N/B6J or Nlrp12B6J/B6J) (n=29, B6N; n=27, B6J; n=23, F1; n=73, F2). (f) Nucleotide sequence profile of the Nlrp12 allele in B6N and B6J mice. The highlighted text indicates the presence of a G to A missense polymorphism in C57BL/6J mice leading to the change of an arginine for lysine at position 1034 of NLRP12. *P<0.05, **P<0.01, ***P<0.005, NS, not significant by Mann–Whitney U-test (a,b,d,e) or log-rank test (c).