Abstract
Introduction
Percutaneous aortic valve replacement (transcatheter aortic valve implantation (TAVI)) notably increases the likelihood of the appearance of a complete left bundle branch block (LBBB) by direct lesion of the LBB of His. This block can lead to high-grade atrioventricular conduction disturbances responsible for a poorer prognosis. The management of this complication remains controversial.
Method and analysis
The screening of LBBB after TAVI persisting for more than 24 hours will be conducted by surface ECG. Stratification will be performed by post-TAVI intracardiac electrophysiological study. Patients at high risk of conduction disturbances (≥70 ms His–ventricle interval (HV) or presence of infra-Hisian block) will be implanted with a pacemaker enabling the recording of disturbance episodes. Those at lower risk (HV <70 ms) will be implanted with a loop recorder device with remote monitoring of cardiovascular implantable electronic devices (CIEDs). Clinical, ECG and implanted device follow-up will also be performed at 3, 6 and 12 months. The primary objective is to assess the efficacy and safety of a decisional algorithm based on electrophysiological study and remote monitoring of CIEDs in the prediction of high-grade conduction disturbances in patients with LBBB after TAVI. The primary end point is to compare the incidence (rate and time to onset) of high-grade conduction disturbances in patients with LBBB after TAVI between the two groups at 12 months. Given the proportion of high-grade conduction disturbances (20–40%), a sample of 200 subjects will allow a margin of error of 6–7%. The LBBB–TAVI Study has been in an active recruiting phase since September 2015 (21 patients already included).
Ethics and dissemination
Local ethics committee authorisation was obtained in May 2015. We will publish findings from this study in a peer-reviewed scientific journal and present results at national and international conferences.
Trial registration number
NCT02482844; Pre-results.
Keywords: Left bundle branch block, remote monitoring., Transcatheter aortic valve implantation, cardiac electrophysiology
Strengths and limitations of this study.
This is the first prospective study designed to evaluate the prognosis of left bundle branch block (LBBB) after transcatheter aortic valve implantation (TAVI).
The use of remote monitoring incorporated into loop recorder and pacemaker devices with the ability to store episodes will enable a better understanding to be achieved.
The LBBB–TAVI Study is a multicentre study involving teams with great experience in TAVI and cardiac rhythm management.
Introduction
The use of percutaneous aortic valve replacement (also known as transcatheter aortic valve implantation (TAVI)), which is reserved for high-risk patients and those with very high operative risk, is rapidly expanding. Its indications tend to extend to intermediate-risk patients. Improvement in valve implantation techniques, the wide dissemination of the procedure, operator experience and the development of new prostheses have dramatically reduced the rate of severe complications.
However, the incidence of a de novo left bundle branch block (LBBB) remains very high, ranging from 5% to 40% depending on the type of valve used.1–3 It is one of the most common complications of this procedure. LBBB is a marker of poor prognosis, with an increased risk of complete atrioventricular (AV) block, heart failure and sudden cardiac death. Implantation of a pacemaker is performed during follow-up in 13.9–20% of LBBBs after TAVI.4 The management of patients with LBBB after TAVI differs from centre to centre because of the absence of strict recommendations by international guidelines. No study to date has allowed definition of the predictors of the occurrence of complete AV block or sudden cardiac death in a population of patients with LBBB after TAVI.
The quality of infra-Hisian conduction in patients with a bundle branch block is evaluated by intracardiac electrophysiological study (EPS), notably by measuring the His–ventricle (HV) interval. Certain studies conducted in patients with syncope in a non-TAVI context noted a relationship between a long HV interval and the risk of syncope or sudden cardiac death.5 As a result, the implantation of a pacemaker in patients with bundle branch block in the case of syncope associated with an HV interval >70 ms and in the presence of an HV interval >100 ms if the patient is asymptomatic is recommended. QRS enlargement after a TAVI procedure, mostly related to new-onset LBBB, is a strong predictive factor of pacemaker implantation.6 Prolongation of intracardiac conduction times during EPS has been identified during and after TAVI implantation procedures.3 7–9 However, a strategy using EPS and remote monitoring of cardiovascular implantable electronic devices (CIEDs) in patients with an asymptomatic conduction disturbance (LBBB) after TAVI has yet to be validated.
Methods and analysis
Objectives
Primary objective
To assess the efficacy and safety of a decisional algorithm based on EPS and remote monitoring of CIEDs to (1) predict the occurrence of high-grade conduction disturbances at 12 months in patients with an LBBB after TAVI and (2) study the tolerance of this management approach combining electrophysiological investigation and implantation of a medical monitoring device (loop recorder or implantable pacemaker).
Secondary objectives
To perform a prognostic stratification of LBBB after TAVI (predictive value of LBBB for mortality (all causes, cardiovascular and heart failure), hospitalisations (all causes, cardiovascular and heart failure) and occurrence of high-grade conduction disturbances at 3 and 6 months)) based on early EPS assessment
To define the predictors of high-grade conduction disturbances in instances of de novo LBBB (clinical, ECG and echocardiographic variables, etc)
To determine the validity of the HV interval in predicting the risk of syncope, complete AV block or sudden cardiac death in this subgroup of patients
End points
Primary end point
Occurrence (rate and time to onset) at 12 months of (1) high-grade conduction disturbances (complete AV block and second-degree Mobitz II AV block in patients with de novo LBBB after TAVI) and (2) adverse events attributable to devices or management: % of haematoma and bleeding, infection (local or systemic), death, hospitalisation, complications related to EPS and vascular access, arrhythmia (supraventricular and ventricular) and stroke.
Secondary end points
Rehospitalisation for discomfort, syncope and implantation of a pacemaker (high-grade conduction disorder, symptomatic sinus node dysfunction, pause >3 s (6 s during night))
Rehospitalisation for heart failure
All-cause mortality
Cardiovascular mortality: myocardial infarction, heart failure, stroke, sudden death
Quality of life assessed by the EuroQol Five Dimensions Questionnaire (EQ-5D) score
Trial inclusion/exclusion criteria
Inclusion criteria
Patient over 18 years of age
Patient implanted with a percutaneous aortic biological valve according to the recommendations of the European Society of Cardiology defined in 201210
Anticipated life expectancy >1 year
Sinus rhythm and atrial fibrillation
Patient with a persistent de novo LBBB after TAVI (>24 hours) and observed during the 7 days after the TAVI procedure
Exclusion criteria
Presence of a pacemaker before TAVI
Presence of an LBBB before TAVI
Pregnancy
No affiliation to a social security scheme
Refusal to participate
Incapacity
Adult under legal protection (trusteeship, guardianship)
Study protocol
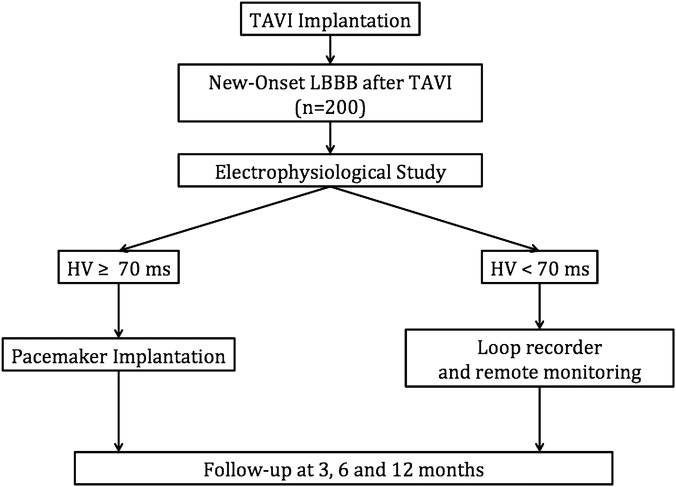
Patients meeting the inclusion criteria, admitted to each cardiology department (University Hospital, Clermont-Ferrand, France; University Hospital, Bordeaux, France; CH Annecy Genèvois, France; Institut Mutualiste Montsouris, France; University Hospital, Saint-Etienne, France; University Hospital, Grenoble, France; Clinique CLAIRVAL, Marseille, France; University Hospital, Toulouse, France) for TAVI implantation, will be screened. Eligible patients will receive a clear verbal explanation and a written information sheet. Finally, written consent will be obtained from each patient (records stored by the promoter in a dedicated archives room) (figure 1).
Figure 1.
Flow diagram of the left bundle branch block–transcatheter aortic valve implantation (LBBB–TAVI) study.
LBBB after the TAVI procedure will be diagnosed by a 12-lead ECG performed daily until the seventh day after implantation. The definition of LBBB is that stipulated by the American Heart Association.11 A clinical examination will be performed. The EQ-5D score assesses the psychometric status (five questions: self-qualitative evaluation of mobility, autonomy, domestic habits, pain and anxiety). It is a generic measure of health status that provides a simple descriptive profile and a single index value that can be used in a population health survey.
In the event of occurrence of an LBBB persisting for more than 24 hours (ie, absence of LBBB before the procedure), an intracardiac EPS will be performed via the femoral venous access, under local anaesthesia between D1 and D4 after TAVI implantation. Two quadripolar leads will allow the collection of atrio-Hissian (AH) and HV intervals and the Wenckebach point. In patients with atrial fibrillation, only the HV interval measurement will be performed, since atrial pacing is not feasible. Patients with a long HV interval will be implanted with a dual-chamber (or single-chamber in the case of atrial fibrillation) pacemaker allowing the recording and quantification of episodes of complete AV block. Patients with a normal HV interval will be implanted with an implantable loop recorder, which also allows the recording of this type of episode. The pacemaker or loop recorder implantation is performed between D3 to D5 after a decrease in inflammatory markers (C-reactive protein) in order to reduce the risk of infection.
Two groups will be defined according to the HV interval measurement.
1. If HV ≥70 ms or in the event of an infra-Hisian block (present if duplication of the Hisian potential >30 ms, prolongation of the HV or non-conducted atrial impulses on atrial ECG followed by a Hisian potential during atrial pacing, Wenckebach point <130 beats/min)
Based on literature data in patients with bundle branch block associated with syncope in a non-TAVI context, this patient group is a priori at very high risk of high-grade conduction disturbances.12 For this reason, a Sorin Group pacemaker, single or dual chamber (Sorin Group CRM, SAS, REPLY 200 or KORA 200/250), will be implanted. This pacemaker will be conventionally implanted according to the usual practice of each operator. One or two leads will be implanted (one in the right atrium and the other in the right ventricle). The type and location of each lead will be left to the discretion of each individual clinician. The pacemaker will be programmed in VVI mode (40 beats/min, backup pacing mode in the event of atrial fibrillation) or AAI-SafeR mode (or AAI-SafeRR mode in the event of an associated sinus node dysfunction). The AAI-SafeR mode allows the maximum rate of right ventricular pacing to be reduced.13 Lastly, the importance of these Sorin prostheses is their ability to store episodes of high-grade conduction disturbances as well as the percentage of right ventricular pacing. The pacemaker can switch from AAI-SafeR mode to DDD mode according to the following criteria: two consecutive blocked P waves (classified as third-degree AV block), three non-consecutive blocked P waves over 12 cycles (classified as second-degree AV block), prolongation of the AV delay (programmed at 350 ms in this study) during six cycles (classified as first-degree AV block) and longer ventricular pauses between 2 and 4 s (programmed at 2 s in this study, classified as a pause). A return to AAI-SafeR mode is regularly tested to avoid prolonged right ventricular pacing (every 100 cycles; less frequently in the event of repeated episodes or if 12 spontaneous cycles detected). These memorised episodes will also allow the rate and time of onset of high-grade conduction disturbances to be ascertained.
2. If HV <70 ms
Based on literature data in patients with bundle branch block associated with syncope in a non-TAVI context, this group is at low risk of high-grade conduction disturbances. A Biomonitor subcutaneous loop recorder (Biotronik, Berlin, Germany) will be implanted—in the left subclavian position for patients implanted with the first-generation Biomonitor (used at the start of the study), and thereafter in the left parasternal position for patients implanted with the second-generation Biomonitor, which will be used starting at the end of 2015. The parameters will be programmed in a homogeneous manner: bradycardia alert when the heart rate is <40 beats/min and the occurrence of a pause >2 s.
Patient follow-up
The clinical follow-up of patients with a pacemaker will be conducted at 3, 6 and 12 months after pacemaker implantation. Patients implanted with a loop recorder will be monitored by remote monitoring of CIEDs (possibility of programmable daily bradycardia alerts with a heart rate <40 beats/min for 10 s and pauses >2 s) combined with a clinical follow-up at 3, 6 and 12 months. This remote monitoring of CIEDs will allow a swift and effective response to be obtained for these patients, which will maximally reduce the risk of complication.
Clinical and functional status, as well as a 12-lead ECG, will also be collected at the various follow-up time points. Transthoracic echocardiography will be performed at 6 and 12 months. The memory banks of the implanted devices will be interrogated (rate and time to onset of conduction disturbance episodes). All data to be collected during this study are summarised in table 1.
Table 1.
Data to be collected as part of the LBBB–TAVI Study
| Variable | Baseline | 3 months | 6 months | 12 months |
|---|---|---|---|---|
| Clinical examination and quality of life questionnaire (EQ-5D) | ✓ | ✓ | ✓ | ✓ |
| ECG before TAVI implantation | ✓ | |||
| TTE | ✓ | ✓ | ✓ | |
| TAVI procedure | ✓ | |||
| ECG after TAVI | ✓ | ✓ | ✓ | ✓ |
| EPS | ✓ | |||
| Pacemaker interrogation | ✓ | ✓ | ✓ | |
| Implantable loop recorder interrogation | ✓ | ✓ | ✓ |
EPS, electrophysiological study; EQ-5D, EuroQol Five Dimensions Questionnaire; LBBB–TAVI, left bundle branch block–transcatheter aortic valve implantation; TTE, transthoracic echocardiography.
The following data will be collected at baseline:
Clinical examination, clinical history (myocardial infarction; coronary artery bypass graft; angioplasty; previous aortic valve dilation; vascular disease; cardiac risk factors), quality of life (EQ-5D), Euroscore
12-lead ECG before TAVI (sinus rhythm; QRS morphology; heart rate (beats/min); PR, QRS, RR, QT, QTc duration (ms))
Transthoracic echocardiography (systolic/diastolic left ventricular (LV) volume; biplane left ventricular ejection fraction (LVEF); left ventricular end-diastolic pressure (LVEDP); diastolic dysfunction (grade); mitral regurgitation (grade); left ventricular outflow tract (LVOT) diameter; aortic annulus; aortic mean gradient; aortic Vmax; aortic calcifications; tricuspid regurgitation (grade); pulmonary artery pressure (PAP))
TAVI implantation (valve type and size; surgical approach route; position of the prosthesis; predilatation balloon size; depth of the prosthesis in outflow tract; per-procedure complication)
Daily 12-lead ECG after TAVI until discharge (at least D7) (conduction disturbances: AV, intraventricular; heart rate (beats/min); PR, QRS, RR, QT, QTc duration (ms); evolution of conduction disturbances; supraventricular or ventricular rhythm disorders; time of onset and persistence of LBBB)
The following data will be collected at 3, 6 and 12 months:
Clinical examination (New York Heart Association functional classification (NYHA); syncope; quality of life (EQ-5D); post-implantation complications (haematoma, lead displacement, local infection, systemic infection, re-intervention, removal of devices, tamponade); hospitalisation (for cardiovascular causes, all causes, heart failure); death (cardiovascular causes, all causes, heart failure); atrial fibrillation)
12-lead ECG before TAVI (sinus rhythm; QRS morphology; heart rate (beats/min); PR, QRS, RR, QT, QTc duration (ms)) and evolution of conduction disturbances: new/recovery LBB, left anterior fascicular block, left anterior hemiblock, right bundle branch block
Transthoracic echocardiography (at 6 and 12 months) (systolic/diastolic LV volume; biplane LVEF; LVEDP; diastolic dysfunction (grade); mitral regurgitation (grade); LVOT diameter; aortic annulus; aortic mean gradient; aortic Vmax; aortic calcifications; tricuspid regurgitation (grade); PAP)
If implanted with a pacemaker (percentage of pacing pulses per chamber; episodes of conduction disturbances (number, time to onset, type); atrial fibrillation, ventricular tachycardia or ventricular fibrillation episodes)
If implanted with a loop recorder (remote monitoring alert (types, number); episodes of conduction disturbances (number, time to onset, type)) and if a pacemaker was implanted and for what reasons.
Calculation of sample size
This protocol could be considered an upstream study of a larger study. It is essential to assess the occurrence of high-grade conduction disturbances at 1 year in patients with de novo LBBB after TAVI. Proposing an accurate sample size estimation appeared difficult a priori based on literature considerations. This type of survey, conducted on a sample population, should allow generalisation of the results to the entire targeted population. Moreover, sample size is a major consideration: the larger the sample, the greater the relevance. In addition to the proportion of events of the primary outcome (high-grade conduction disturbances), an error margin of the estimate has been fixed. Given a proportion of high-grade conduction disturbances ranging between 20% and 40%, a sample size of 200 subjects will allow a margin of error of 6–7%. This sample size (n=200) is deemed sufficient to address the objective of determining the prognostic factors of high-grade conduction disturbances in the event of de novo LBBB14 with satisfactory statistical power. No particular consideration will be given to sample size estimation associated with the safety objective. Each patient presenting with an adverse event will be investigated by the Data Monitoring Safety Committee who will decide (1) the accountability of the device or treatment and (2) whether the study should be continued.
Statistical analysis
Statistical analysis will be performed with Stata software. The tests will be two-sided, with a type I error set at α=0.05. The epidemiological, clinical and biological characteristics as well as treatment will be analysed as follows: quantitative variables will be presented as mean (SD) or median (IQR) according to the statistical distribution (assumption of normality will be assessed using the Shapiro-Wilk test). To evaluate time to occurrence of high-grade conduction disturbances, censored data analyses will be considered. Time intervals will not be determined by observed dates of events but by fixed intervals. The estimation of time to occurrence of high-grade conduction disturbances will be performed by an actuarial method. The safety analysis will be primarily descriptive. Each patient presenting with an adverse event will be studied independently by the Data Monitoring Safety Committee. To determine prognostic factors of high-grade conduction disturbances, the following statistical analysis scheme is proposed. Univariate analysis will be performed using a log-rank test for categorical variables and a Cox proportional hazards model for quantitative outcomes. When appropriate, quantitative variables will be categorised according to clinical relevance and statistical distribution and after studying the receiver operating characteristic (ROC) curve and estimating several of the usual indices (Youden, Liu, efficiency and grey-zone). In multivariate analysis, Cox regression models will be performed with covariates fixed according to univariate results and clinical relevance. The interactions between prognostic factors will be studied. The proportional-hazards hypothesis will be verified using Schoenfeld's test and plotting residuals. Particular focus will be given to the study of multicollinearity. The possible centre effect will be considered as a random effect using marginal Cox models. According to the note proposed by Adams and Leveson,15 a prediction model based on multivariate analysis will be proposed. The factors shown to be significantly related to the outcome in this observational study will be allocated a score (‘weight’). The cumulative final score of all the risk factors present in a patient will be used as an indicator of the likelihood of the outcome occurring. Results will be expressed as HRs and 95% CI. The final model will be validated by a two-step bootstrapping process. In each step, 1000 bootstrap samples with replacements will be created from the training set. In the first step, using the stepwise procedure, the percentage of models including each of the initial variables will be determined. In the second step, the Cox regression parameters of the final model will be independently estimated. The bootstrap estimates of each covariate coefficient and SE will be averaged from these replicates. Log-likelihood will be measured by the goodness-of-fit of a model. After these multivariate analyses, a ROC curve will be plotted for the final model, and the area under the curve will be estimated. A score predicting the occurrence of high-grade conduction disturbances (prognostic score) will be estimated according to HR values. The threshold value of this score will be determined according to the usual recommendations by estimating several indices such as Youden, Liu and efficiency. Sensitivity, specificity and negative/positive predictive values will be presented with 95% CIs. The analyses described above will also be applied to other end points (overall survival, hospitalisation for all causes and hospitalisation for cardiovascular causes). Thereafter, the usual statistical tests will be performed to compare independent groups (eg, HV < or ≥70 ms): Student's t test or Mann-Whitney test if the conditions of the t test are not met (normality, homoscedasticity analysed by the Fisher-Snedecor test) for quantitative variables, and χ2 or Fisher's exact tests for categorical variables. Given that observational studies preclude making conclusions in terms of causality, the use of propensity scores developed by Rosenbaum and Rubin (1983; http://biomet.oxfordjournals.org/content/70/1/41) may be proposed. Finally, repeated longitudinally collected data (including EQ-5D scores) will be analysed using random-effects models taking into account within- and between-subject variability. As proposed by several statisticians, all individual p values will be reported without systematically performing a mathematical correction for distinct tests.16 Particular focus will be given to the magnitude of difference and to clinical relevance.17 A sensitivity analysis will be proposed in order to study the statistical nature of the missing data and to propose the most appropriate approach to imputation of missing data.
Data Monitoring Safety Committee
A Data Monitoring Safety Committee (independent of the sponsor) will meet initially at the launch of the study and thereafter throughout the study, on its own initiative or at the request of the sponsor, and will discuss the results of the intermediate analyses. It will have a consultative role. It will provide a general opinion on the progress of the study. It may assist in the making of difficult decisions during the course of the study for which an independent judgement is desirable. The Data Monitoring Safety Committee will decide on possible early termination in the light of data transmitted by the adjudication committee, the role of which will be defined by the Data Monitoring Safety Committee.
Auditing will be performed using eCRF Cleansight independently of investigator and sponsor intervention.
Ethics and dissemination
The LBBB–TAVI Study has been in an active recruiting phase since September 2015 (21 patients already included). Local ethics committee (CPP Sud Est VI, AU1181) and ANSM (Agence Nationale de sécurité du medicament et des produits de santé, 2015-A00271–48) authorisation was obtained in May 2015. The study completion date is estimated to be September 2018. This multicentre, randomised, parallel-group trial is registered at http://www.clinicaltrials.gov under the registration number NCT02482844.
We will publish findings from this clinical study in a peer-reviewed scientific journal and present results at national and international conferences.
Discussion
The appearance of an LBBB after TAVI is a common complication which raises several questions. (1) Which treatment option should be used for LBBB after TAVI? (2) What is the future for these patients? (3) How should patients at risk of developing high-grade conduction disturbances be identified?
1. Which treatment option for LBBB after TAVI?
The unpredictable course of LBBB renders its management difficult given the absence to date of recommendations by scientific societies. There is therefore no standardised management. Several options are currently available.
A wait-and-see attitude by simple clinical monitoring can lead to serious complications.
The systematic implantation of a pacemaker is the preferred solution for many centres but can lead to short- and long-term complications. Indeed, systematic implantation can lead to a risk of infection in patients with a prosthetic valve, who are often frail and with many comorbidities. In the case of expansion of indications to younger patients with fewer comorbidities, the question arises as to the impact of chronic right ventricular pacing. In the case of pacemaker implantation, the type of device and pacing mode remain debatable and should be subjected to dedicated studies.
Thus, the use of EPS stratification and implantable monitoring combined with remote monitoring of CIED follow-up represents an interesting option for documenting and managing a high-grade conduction disturbance as early as possible. This strategy has the advantage of only implanting a pacemaker in those patients in whom there is an absolute necessity.
Lastly, the establishment of indications for TAVI procedures in intermediate-risk patients appears to be a major and pressing perspective of this technique. The increase in the prevalence of abnormal conduction is a considerable limitation of this technique, particularly in younger patients with fewer comorbidities.
2. What is the future for these patients?
The appearance of a persistent LBBB can lead to rhythmic and haemodynamic complications. Although the majority of conduction disturbances occur early—that is, in the first week after implantation (90% of cases)—they can also occur later and carry the risk of syncope and sudden death. LBBB persists in two out of three patients at 1 month and its evolution is difficult to predict. Indeed, de novo LBBB may be transient, but may also appear up to 1 year after the procedure (0.8% of patients).18–21 LBBB after TAVI can lead to symptomatic heart failure especially in the presence of pre-existing left ventricular dysfunction. Indeed, the presence of LBBB after TAVI in this frail elderly population decreases survival at 1 year (3.3% vs 13% p=0.014).22 The presence of an LBBB after TAVI or the implantation of a pacemaker reduces functional improvement but also the improvement in LVEF expected after a valve replacement.23 The impact on survival remains controversial,20 24 although the presence of an LBBB imposes the risk of sudden death.
Expansion of indications of TAVI to younger, intermediate-risk patients requires that we extend our knowledge of LBBB after TAVI. Beyond the technological advancements that enable the rate of LBBB to be reduced (new valve types, new implantation methods, operator experience), its management must be clarified, and the potential clinical application of our prognostic decision tree in intermediate-risk TAVI candidates necessitates further research. In order to achieve this, we first need to define the patients at high risk of rhythmic complications.
3. How to identify patients at risk of developing high-grade conduction disturbances?
The LBBB–TAVI Study is dedicated to determining the occurrence of conduction disturbances in the event of LBBB after TAVI according to a stratification of the risk of occurrence of high-level AV block. Intracardiac electrophysiology is able to identify patients at most risk, who require implantation of a double-chamber pacemaker as first-line treatment. Measuring the HV interval completes the surface EG data and objectifies the presence of an infra-Hisian lesion at high risk of AV block, demonstrated only in a small cohort of patients.3 25 The predictive value of the HV interval has not been validated in this very specific setting.
In addition, all of the collated clinical, ECG (LBBB width, persistence, time to onset, etc) and procedural data will enable the identification of patients at high risk of conduction disturbances. An ECG at baseline and during follow-up offers a first assessment of conduction disorders or abnormal QRS morphology associated with pacemaker implantation. The latter may represent confounding factors and will be evaluated.
Lastly, the accurate and dated collection of the occurrence of abnormal AV conduction will be possible because of the previously described algorithms of the Sorin Group pacemakers as well as by daily remote monitoring of the implantable loop recorder. Remote monitoring of CIEDs is a major innovation and constitutes a breakthrough in the monitoring and management of patients with pacemakers or defibrillators. As an integral part of our study, remote monitoring will allow the collection of episodes, asymptomatic or not, of abnormal conduction (bradycardia, pauses) and early treatment if needed.
Conclusion
The LBBB–TAVI Study has the novel initiative of studying the incidence and predictors of high-grade conduction disturbances in patients with LBBB after TAVI. The expansion of indications for TAVI and the international distribution of this procedure provide great incentive to broaden our knowledge and orient the future of the management of de novo LBBB during TAVI in high-risk patients and may require further research in intermediate-risk groups.
Acknowledgments
We thank Mr Pierre Pothier for editing this manuscript and Miss Elodie Chazot for daily coordination of the NICD-CRT study.
Footnotes
Contributors: GM, FJ, GS, BP and RE contributed to the conception of the study. All authors contributed to the study design. The manuscript protocol was drafted by GM, BP, PB and RE. Screening of studies for inclusion will be undertaken by all authors (clinician) except BP (methodologist). Data extraction will be undertaken by GM, BP and RE. All authors approved the publication of the protocol.
Funding: This clinical trial is funded with the support of Biotronik and the Sorin Group. However, the authors are solely responsible for the design and conduct of this study as well as all study analyses and the drafting and editing of the article.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Local ethics committee (CPP Sud Est VI, AU1181).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Erkapic D, De Rosa S, Kelava A et al. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol 2012;23:391–7. 10.1111/j.1540-8167.2011.02211.x [DOI] [PubMed] [Google Scholar]
- 2.Sinhal A, Altwegg L, Pasupati S et al. Atrioventricular block after transcatheter balloon expandable aortic valve implantation. JACC Cardiovasc Interv 2008;1:305–9. 10.1016/j.jcin.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 3.Akin I, Kische S, Paranskaya L et al. Predictive factors for pacemaker requirement after transcatheter aortic valve implantation. BMC Cardiovasc Disord 2012;12:87 10.1186/1471-2261-12-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urena M, Webb JG, Cheema A et al. Impact of new-onset persistent left bundle branch block on late clinical outcomes in patients undergoing transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv 2014;7:128–36. 10.1016/j.jcin.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 5.Scheinman MM, Peters RW, Suavé MJ et al. Value of the H-Q interval in patients with bundle branch block and the role of prophylactic permanent pacing. Am J Cardiol 1982;50:1316–22. 10.1016/0002-9149(82)90469-6 [DOI] [PubMed] [Google Scholar]
- 6.Khawaja MZ, Rajani R, Cook A et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative). Circulation 2011;123:951–60. 10.1161/CIRCULATIONAHA.109.927152 [DOI] [PubMed] [Google Scholar]
- 7.Rubín JM, Avanzas P, del Valle R et al. Atrioventricular conduction disturbance characterization in transcatheter aortic valve implantation with the CoreValve prosthesis. Circ Cardiovasc Interv 2011;4:280–6. 10.1161/CIRCINTERVENTIONS.111.961649 [DOI] [PubMed] [Google Scholar]
- 8.Akin I, Kische S, Schneider H et al. Surface and intracardiac ECG for discriminating conduction disorders after CoreValve implantation. Clin Res Cardiol 2012;101:357–64. 10.1007/s00392-011-0400-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eksik A, Gul M, Uyarel H et al. Electrophysiological evaluation of atrioventricular conduction disturbances in transcatheter aortic valve implantation with Edwards SAPIEN prosthesis. J Invasive Cardiol 2013;25:305–9. [PubMed] [Google Scholar]
- 10.Vahanian A, Alfieri O, Andreotti F et al. with Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–96. 10.1093/eurheartj/ehs109 [DOI] [PubMed] [Google Scholar]
- 11.Surawicz B, Childers R, Deal BJ et al. , American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation, Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:976–81. 10.1016/j.jacc.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 12.Brignole M, Auricchio A, Baron-Esquivias G et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–329. 10.1093/eurheartj/eht150 [DOI] [PubMed] [Google Scholar]
- 13.Stockburger M, Boveda S, Moreno J et al. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J 2015;36:151–7. 10.1093/eurheartj/ehu336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 15.Adams ST, Leveson SH. Clinical prediction rules. BMJ 2012;344:d8312 10.1136/bmj.d8312 [DOI] [PubMed] [Google Scholar]
- 16.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 17.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002;2:8 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazif TM, Dizon JM, Hahn RT et al. , PARTNER Publications Office. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv 2015;8:60–9. 10.1016/j.jcin.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 19.Boerlage-Van Dijk K, Kooiman KM, Yong ZY et al. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol 2014;37:1520–9. 10.1111/pace.12460 [DOI] [PubMed] [Google Scholar]
- 20.Testa L, Latib A, De Marco F et al. Clinical impact of persistent left bundle-branch block after transcatheter aortic valve implantation with CoreValve Revalving System. Circulation 2013;127:1300–7. 10.1161/CIRCULATIONAHA.112.001099 [DOI] [PubMed] [Google Scholar]
- 21.Houthuizen P, van der Boon RMA, Urena M et al. Occurrence, fate and consequences of ventricular conduction abnormalities after transcatheter aortic valve implantation. EuroIntervention 2014;9:1142–50. 10.4244/EIJV9I10A194 [DOI] [PubMed] [Google Scholar]
- 22.Schymik G, Tzamalis P, Bramlage P et al. Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin Res Cardiol 2015;104:351–62. 10.1007/s00392-014-0791-2 [DOI] [PubMed] [Google Scholar]
- 23.Urena M, Webb JG, Tamburino C et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233–43. 10.1161/CIRCULATIONAHA.113.005479 [DOI] [PubMed] [Google Scholar]
- 24.Urena M, Mok M, Serra V et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J Am Coll Cardiol 2012;60:1743–52. 10.1016/j.jacc.2012.07.035 [DOI] [PubMed] [Google Scholar]
- 25.Shin DI, Merx MW, Meyer C et al. Baseline HV-interval predicts complete AV-block secondary to transcatheter aortic valve implantation. Acta Cardiol 2015;70:574–80. 10.2143/AC.70.5.3110518 [DOI] [PubMed] [Google Scholar]