Abstract
SETTING
Rwanda has generalised human immunodeficiency virus (HIV) and tuberculosis (TB) epidemics. The Rwandan Ministry of Health approved a policy on TB-HIV collaborative activities in 2005. The present study is a report on the results of the integrated TB and HIV activities at a rural health care site between July 2005 and June 2006.
METHODS
Activities included provider-initiated HIV testing and counselling (PITC) of TB patients and the implementation of a standardised TB screening questionnaire for in-patients on medical wards and HIV-infected out-patients.
RESULTS
Of a total 259 TB patients registered, 87% with unknown HIV status or who were HIV-negative accepted PITC. Overall, 48% (125/259) of TB patients were HIV-infected. The proportion of TB patients ever tested for HIV increased from 82% (138/169) in 2004–2005 to 93% (240/259) in 2005–2006 (P < 0.001). Of the 770 in-patients screened for TB, 162 (21%) tested positive, of whom 53 (33%) were diagnosed with TB; 39 (76%) of these were HIV co-infected. Three hundred out-patients with HIV were screened for TB; 80 (27%) tested positive, of whom 11 (14%) were diagnosed with TB.
DISCUSSION
Activities integrating TB and HIV were feasible in a rural health care setting. PITC was successful in TB patients and unrecognised TB was common, particularly among HIV-infected in-patients.
Keywords: tuberculosis, human immunodeficiency virus, TB-HIV co-infection, integration
RÉSUMÉ
CONTEXTE
Le Rwanda connaît des épidémies généralisées dues au virus de l’immunodéficience humaine (VIH) et la tuberculose (TB). En 2005, le Ministère de la Santé du Rwanda a approuvé une politique d’activités conjointes TB-VIH. Nous faisons état des résultats des activités intégrées TB et VIH dans un site rural de soins de santé entre juin 2005 et juin 2006.
MÉTHODES
Les activités ont comporté un test et un accompagnement pour le VIH à l’initiative du pourvoyeur de soins (PITC) chez les patients TB et la mise en oeuvre d’un questionnaire standardisé de dépistage de la TB pour les patients hospitalisés dans les salles de médecine et pour les patients externes infectés par le VIH.
RÉSULTATS
On a enregistrés 259 patients TB. Le PITC a été accepté par 87% des patients TB dont le statut VIH était inconnu ou négatif. Au total, 48% des patients TB (125/259) étaient infectés par le VIH. La proportion de patients TB ayant été testés pour le VIH à un moment quelconque a augmenté depuis 82% (138/189) en 2004– 2005 jusqu’à 93% (240/259) en 2005–2006 (P < 0,001). Des 770 patients hospitalisés dépistés pour la TB, 162 (21%) se sont avérés positifs par le questionnaire et 53 de ceux-ci (33%) ont été diagnostiqués comme atteints de TB dont 39 (76%) étaient co-infectés par le VIH. On a testé pour la TB 300 patients ambulatoires infectés par le VIH, dont 80 (27%) ont été dépistés comme positifs par le questionnaire et parmi eux 11 (14%) comme atteints de TB.
DISCUSSION
Les activités intégrant VIH et TB sont réalisables dans un contexte rural de soins de santé. Le PITC a été couronné de succès chez les patients TB ; la TB méconnue a été courante, particulièrement chez les patients hospitalisés infectés par le VIH.
RESUMEN
MARCO DE REFERENCIA
En Ruanda, se presencian epidemias generalizadas de infección por el virus de la inmunodeficiencia humana (VIH) y de tuberculosis (TB). El ministerio ruandés de la salud aprobó una política de actividades conjuntas contra TB y VIH en 2005. En el presente artículo, se presentan los resultados de estas actividades integradas en un centro rural de salud entre julio de 2005 y junio de 2006.
MÉTODOS
Las actividades comprendieron prueba diagnóstica y orientación por el VIH por iniciativa del proveedor de salud (PITC) a los pacientes con TB y administración de un cuestionario normalizado de detección de la TB a los pacientes hospitalizados en servicios médicos y a los pacientes en manejo ambulatorio de la infección por el VIH.
RESULTADOS
Se registraron 259 pacientes tuberculosos. Ochenta y siete por ciento de los pacientes con TB que ignoraban el estado de su serología del VIH aceptaron la PITC propuesta. Globalmente, 48% (125/259) de los pacientes tuberculosos presentaron infección por el VIH. La proporción de pacientes con TB y antecedente de pruebas del VIH aumentó de 82% (138/169) entre 2004 y 2005 a 93% (240/259) entre 2005 y 2006 (P < 0,001). De los 770 pacientes hospitalizados en quienes se practicó la detección sistemática de TB, 162 (21%) tuvieron un resultado positivo y en 53 (33%) de estos se confirmó el diagnóstico ; 39 (76%) de los pacientes con TB confirmada presentaron coinfección por el VIH. Se practicó la detección sistemática de TB en 300 pacientes ambulatorios infectados por el VIH : en 80 (27%) la detección fue positiva y se confirmó el diagnóstico de TB en 11 (14%) de ellos.
CONCLUSIÓN
Se demostró la factibilidad de realizar actividades conjuntas contra la TB y la infección por el VIH en un centro rural de salud. La PITC en pacientes con TB fue exitosa y con frecuencia se detectaron casos de TB hasta el momento inadvertidos, en particular en pacientes hospitalizados con infección por el VIH.
Tuberculosis (TB) is the leading killer of human immunodeficiency virus (HIV) infected individuals, and the overlapping epidemics have had a devastating impact on TB and HIV morbidity, mortality and control worldwide. Rwanda has a generalised HIV epidemic, with 3% of the adult population living with HIV/AIDS in 2005.1 The World Health Organization (WHO) estimated Rwanda’s TB incidence in 2003 to be 371 per 100 000 population.2
In 2003, the Treatment Research AIDS Centre (TRAC) coordinated the national roll-out plan for antiretroviral treatment (ART). The TRAC national plan focuses on comprehensive facility-based HIV care and ART services with strong community-based components to provide ongoing support to patients. By the end of 2006, 133 health facilities were providing HIV care and treatment services.
The DOTS strategy has been uniformly implemented in Rwanda since 1990 through the Programme National Intégré de lutte contre la Lèpre et la Tuberculose (PNILT). In 2003, the treatment success rate for new sputum smear-positive pulmonary TB was 83.2% and in 2004 the detection rate was 48%.3
In 2005, the Rwandan Ministry of Health approved a national policy on TB-HIV collaborative activities4 based on World Health Organization (WHO) policies5 and in line with the WHO’s Stop TB strategy with its enhancement of DOTS and its focus on TB-HIV and the growing problem of multidrug-resistant TB (MDRTB).6 The Rwandan national policy recommends routine (opt-out) provider-initiated HIV testing and counselling (PITC) for all TB patients, the availability of HIV-related services to HIV-infected TB patients, improved active TB disease case finding through standardised screening, and TB suspect evaluations of HIV-infected patients.
In August 2005, an integrated TB-HIV programme was initiated in Gisenyi. The purpose of the programme was to develop, evaluate and share best practices for TB-HIV integrated care using available TB and HIV services. In this communication, we report on the initial results of the programme.
METHODS
Setting
The Gisenyi District Hospital (GDH) and the Gisenyi Health Centre (GHC) are in Gisenyi Province in Rwanda. GDH is a rural public hospital with 308 inpatient beds serving 528 665 individuals in its catchment area. GDH employs a staff of 149 members, including eight physicians and 78 nurses. GDH and GHC are adjacent to each other, with no staff overlap. At GHC, HIV voluntary counselling and testing services (VCT) became available in 2003. In 2005, 5282 individuals were tested through these services. Patients attending GHC have access to TB diagnosis but not TB treatment services.
While VCT is unavailable on-site at GDH, an HIV clinic with care and treatment services, including cotrimoxazole preventive treatment (CPT) and ART, was established in 2004. As of December 2006, the HIV clinic had enrolled 2559 patients, 935 of whom initiated ART. TB diagnostic and treatment services are available on-site at the GDH. TB treatment via directly observed treatment (DOT) is provided free of charge, and on average 110 patients per month receive treatment at the GDH TB clinic, of whom about 10% are hospitalised in a 17-bed TB ward. Thus, while TB treatment and HIV care and treatment services are available at the GDH, VCT is only available at the GHC.
Implementation of TB-HIV integration
Staffing for the integration effort
A nurse was employed in August 2005 for the new position of TB-HIV focal point person. This individual was responsible for overseeing the integration of TB-HIV care and treatment activities at the GDH and the GHC.
Multidisciplinary team meetings
The TB-HIV focal point person and TB nurse attended multi-disciplinary team meetings at the HIV clinic. At these weekly meetings, the team discussed the care and treatment of TB-HIV co-infected patients followed at both the TB and the HIV clinics.
Establishing PITC at the TB clinic
The TB treatment nurse and the TB-HIV focal point person attended training on HIV counselling and testing. After completion of training, they initiated PITC in August 2005 for all TB patients by first piloting it among in-patients (regardless of TB status) at GDH and then focusing this effort on TB patients, including those followed up at the TB clinic. TB patients with unknown HIV status or who were HIV-negative >3 months prior to the start of TB treatment were counselled about HIV testing by the TB nurse or the TB-HIV focal point person during the intensive phase of anti-tuberculosis treatment. Blood sampling for HIV testing was performed in the TB clinic or ward. Samples were transported at the end of the day by the TB nurse to the VCT programme at GHC to perform HIV testing using rapid test kits, and available results were collected. Post-test counselling was performed the next day for TB patients receiving ambulatory DOT or on the same day in the TB ward for in-patients. All TB out-patients found to be HIV-infected using PITC or with prior knowledge of positive HIV status were escorted by the TB-HIV focal point person to the HIV clinic, or a nurse from the HIV clinic came to the TB clinic to enrol the patient into HIV care. ART was initiated if the patient was eligible for treatment as per Rwandan national guidelines. PITC was provided at no cost to all TB patients.
Screening for TB in HIV-infected patients
A TB screening questionnaire developed for use in patients with advanced HIV disease7 was modified to complement current Rwandan TB guidelines and piloted among in-patients at GDH for 4 weeks in November and early December 2005 (Table). The screening questionnaire was then put to the patients in the medical ward at GDH from December 2005 by health care providers as part of routine care for all in-patients, irrespective of HIV status. In April 2006, the questionnaire began to be used routinely at initial and follow-up visits at the HIV clinic at GDH. Patients who screened positive on the questionnaire (i.e., who had an affirmative response to any of the five questions posed) were considered TB suspects and referred for further work-up and evaluation per national guidelines for TB diagnosis.8
Table.
TB screening questionnaire (modified from Mohammed et al.7).
| 1 | Has the patient had a cough for ≥3 weeks? |
| 2 | Has the patient had night sweats for ≥3 weeks? |
| 3 | Has the patient lost ≥3 kg in the past 4 months? |
| 4 | Has the patient had fever for ≥3 weeks? |
| 5 | Has the patient had recent contact with another person with active TB? |
| • | If ‘yes’ to question 1: the patient is a TB suspect, perform sputum collection for AFB smear and continue evaluation for TB per the PNILT diagnostic algorithm for pulmonary TB |
| • | If ‘no’ to question 1 but ‘yes’ to any other question: the patient is a TB suspect; continue evaluation for TB guided by clinical signs and symptoms. Refer to national reference hospital if necessary. |
| • | If ‘no’ to all the questions: the patient is not a TB suspect at this time; stop investigations for TB and repeat screening with questionnaire every 3–6 months. |
TB = tuberculosis; AFB = acid-fast bacilli; PNILT = Programme National Intégré de Lutte contre la Lèpre et la Tuberculose.
Data collection and evaluation of activities
The TB Clinic at GDH has been recording information on the HIV status of TB patients since 2000 in the national TB registers provided by the PNILT. To enhance the data collected on TB patients with HIV co-infection, new monitoring and evaluation tools were developed and implemented as part of routine data collection, including a new TB-HIV register to complement the existing TB registers. These TB-HIV registers captured information about type of TB, HIV status of the patient at the start and end of TB treatment and use of CPT and ART. The new TB-HIV register was first introduced in the beginning of the third quarter (Q3) of 2005 at the GDH TB clinic. The goal of these new TB-HIV registers was to provide information on the continued evolution of the national TB registers by piloting new TB-HIV indicators. HIV patient cards were also modified to capture information on TB screening and diagnosis of TB. Data for the analysis presented below were collected from the national TB register, the new TB-HIV register and HIV patient cards.
Statistical analysis
National TB registry data for the GDH TB clinic from Q3 and Q4 of 2004 and Q1 and Q2 of 2005 (i.e., prior to the implementation of the PITC programme) were compared to those of Q3 and Q4 of 2005 and the first two quarters of 2006 combined. Categorical data were analysed using the χ2 function of Epi Info version 6 (Centers for Disease Control and Prevention, Atlanta, GA, USA). A P value of <0.05 was considered significant.
RESULTS
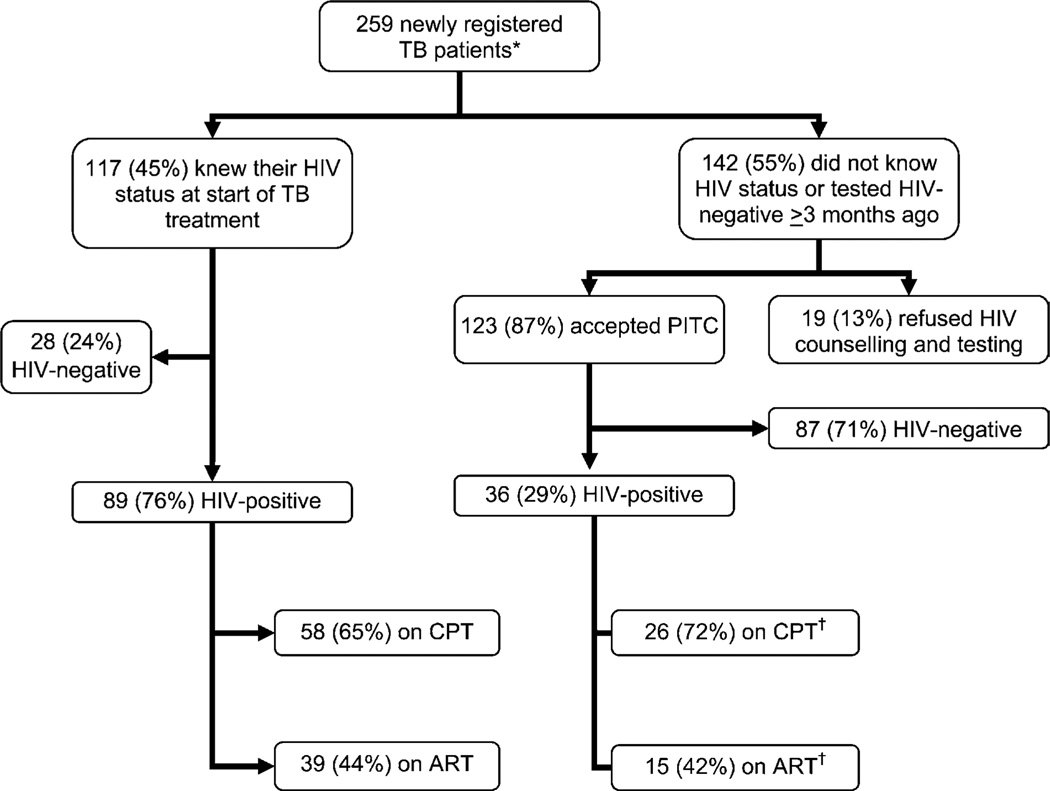
Detailed results of the PITC programme and its link with HIV care in the 259 newly registered TB patients at the GDH TB clinic from the Q3 and Q4 of 2005 and the Q1 and Q2 of 2006 were available. Results are shown in Figure 1. Forty-five per cent of TB patients knew their HIV status at enrolment and 87% of those who were unaware of their HIV status or who were HIV-negative but had last been tested ≥3 months previously accepted PITC. Overall, 48% (125/259) of TB patients were HIV-infected. Patients who knew their HIV status at enrolment were more likely to be HIV-infected than those who did not (odds ratio [OR] 7.68, 95% confidence interval [CI] 4.16–14.27, P < 0.0001). Of those patients newly diagnosed as HIV-infected, 72% were started on CPT and 42% were started on ART by the time continuation phase TB treatment was initiated.
Figure 1.
PITC in newly registered TB patients at the Gisenyi District Hospital TB treatment programme and use of CPT and ART in TB-HIV co-infected patients: beginning of Q3 2005 to end of Q2 2006. *Does not include patients transferred in from other TB treatment programmes.† By the time patient was in the continuation phase of TB treatment. TB = tuberculosis; HIV = human immunodeficiency virus; PITC = provider-initiated HIV testing and counselling; CPT = cotrimoxazole preventive treatment; ART = antiretroviral treatment; Q = quarter.
The proportion of registered TB patients reported to have known HIV status at the GDH TB clinic was 82% (138/129) in the last two quarters of 2004 and the first two quarters of 2005 combined vs. 93% (240/259) in the last two quarters of 2005 and the first two quarters of 2006 combined (P < 0.001). In 2006 as a whole, 97% (282/290) of TB patients at GDH had known HIV status.
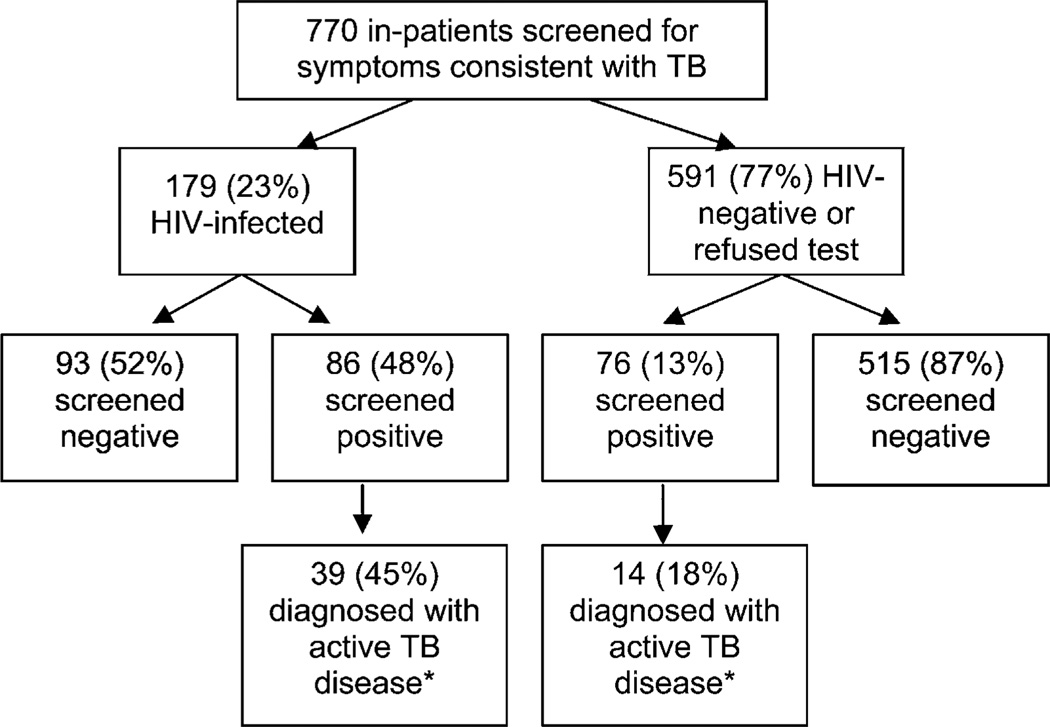
Between December 2005 and June 2006, 770 inpatients were screened for TB using the questionnaire developed for this purpose, of whom 162 (21%) screened positive; of these, 53 were diagnosed as having TB as per national guidelines. Of the 53 patients with TB, 39 (74%) were HIV co-infected (Figure 2). Overall, 7% (53/770) of all in-patients and 22% (39/179) of HIV-infected in-patients on the medical wards were found to have TB through routine use of the questionnaire and evaluation of those who screened positive. Data on the type of TB diagnosed among these in-patients were only collected during the first month of evaluation. In December 2005, 11 patients were diagnosed with TB (9 HIV-positive): one was smear-positive, five were smear-negative and five were extra-pulmonary cases of TB. Between April and June of 2006, 300 of 629 patients attending the HIV clinic were screened for TB at their visits. Eighty patients screened positive, of whom 11 (14%) were diagnosed as having active TB and started treatment.
Figure 2.
Intensified TB screening and active case finding among in-patients at Gisenyi District Hospital, December 2005 to June 2006. *Per Rwandan National TB Control Programme diagnostic algorithms and guidelines for the diagnosis of active TB disease. TB = tuberculosis; HIV = human immunodeficiency virus.
DISCUSSION
Given the high rate of TB-HIV co-infection, HIV and TB require a coordinated approach to diagnosis and treatment. The approach to integration of TB and HIV programmes in Rwanda is based on the philosophy that services should be of high quality, multidisciplinary and draw on the skills and resources of both TB and HIV programmes and providers.
We were able to develop and implement systematic changes in the functions of the TB and HIV programmes and the availability and integration of TB and HIV services for co-infected patients through relatively simple modifications and innovative use of the resources already on-site. A rural site was deliberately chosen to develop an integrated programme based on existing resources, services and infrastructure, making replication of such an approach feasible in similar settings in Rwanda. The only additional resource introduced was the hiring of the new TB-HIV focal point person. Although an added cost to the programme, this individual was crucial for supervising and promoting collaboration between staff members and the integration of the services.
Expediting the training of the TB nurse and the TB-HIV focal point person in HIV CT and linking with the VCT services available at GHC allowed for the successful implementation of PITC. Newly diagnosed HIV-infected patients were successfully enrolled into HIV care and treatment, with three-quarters starting CPT and close to half starting ART. Arguably, these patients may never have been enrolled into care, may have had their enrolment delayed or may have succumbed to unrecognised HIV disease had it not been for the PITC programme.
The TB screening questionnaire was designed for ease of use, and to favour sensitivity over specificity. The questionnaire continues to be used to screen all in-patients and for screening of all HIV-infected outpatients every 3–6 months as part of routine HIV care. The HIV patient medical record has also been modified to prompt providers to utilise questionnaires for TB screening and to record the results of TB screening.
The introduction of a new TB-HIV register allowed for the monitoring and evaluation of more detailed aspects of TB-HIV co-infection care and treatment than was possible with the old national TB registers. As additional registers mean additional paperwork and the potential for confusion and misreporting of data, the key indicators from the TB-HIV register have now been incorporated into newer versions of the national TB register which are being used at TB treatment facilities nationwide.
Limitations
The HIV status of in-patients was largely unknown as these patients had to bear the costs involved. While HIV counselling and testing is available at no cost to patients in VCT settings and for patients with known TB, availability of PITC free of charge to all patients in this and similar settings would allow for HIV diagnosis at health care facilities. We were unable to formally determine the burden of this integration effort, both in terms of staff time devoted to these efforts and in economic terms. Most changes made, except for the hire of the TB-HIV focal point person, were at no additional cost to the programme. It is possible that in some settings the role and responsibilities of the TB-HIV focal point person could be undertaken by an existing staff member.
The operating characteristics of the TB screening questionnaire are unknown. If it proves to have limited specificity, this would lead to excessive evaluations of patients identified as TB suspects who do not have the disease or, if found to have limited sensitivity, it would result in missed TB cases. The design and content of the questionnaire we utilised were based on a similar questionnaire with excellent operating characteristics.7
CONCLUSION
The implementation of a simple TB screening questionnaire led to increased detection of TB, in both hospitalised patients, irrespective of HIV status, as well as HIV patients in an out-patient setting. Implementation of PITC at the TB clinic and the creative integration of the services available on site led to an increase in the proportion of TB patients with known HIV status. We believe these efforts were made feasible by the presence of a TB-HIV focal point person on site to supervise and promote the integration efforts and the establishment of multi-disciplinary team meetings between TB clinic and HIV clinic staff. All of the TB and HIV staff involved were very enthusiastic and motivated from the inception of the programme and throughout its implementation. There was no reluctance on the part of TB staff to implement PITC or on the part of HIV staff to screen for TB.
These integration activities brought the often disparate TB and HIV provider teams together and overcame the erroneous perception of TB and HIV as separate conditions that required separate services rather than as interrelated conditions often occurring in the same patient. Focusing the services for the benefit of the co-infected represents the greatest success of the programme.
Acknowledgments
The authors thank Ms V Koscelnik, formerly of the Centers for Disease Control and Prevention (CDC) Rwanda and Dr J C Karasi at the Rwandan Ministry of Health. This evaluation was supported by CDC/Global AIDS Programme (GAP) Cooperative Agreement U62/CCU223540 and CDC/GAP Cooperative Agreement U62/CCU222407, under the President’s Emergency Plan for AIDS Relief.
References
- 1.Ministry of Health, Rwanda. Kigali, Rwanda: MOH; 2005. [Accessed 29 March 2006]. Demographic and health survey. http://www.moh.gov.rw/press_release_dhs.htm. [Google Scholar]
- 2.World Health Organization. WHO/HTM/TB/2006.362. Geneva, Switzerland: WHO; 2006. WHO report 2006. Global tuberculosis control: surveillance, planning, financing. [Google Scholar]
- 3.Programme National Intégré de lutte contre la Lèpre et la Tuberculose. Annual report. Kigali, Rwanda: Ministry of Health; 2006. [Google Scholar]
- 4.Ministry of Health, Republic of Rwanda. Policy statement on TB-HIV collaborative activities. Kigali, Rwanda: MOH; 2005. [Google Scholar]
- 5.Stop TB Department and Department of HIV/AIDS, World Health Organization. WHO/HTM/TB/2004.330, WHO/HTM/HIV/2004.1. Geneva, Switzerland: WHO; 2004. Interim policy on collaborative TB-HIV activities. [Google Scholar]
- 6.World Health Organization. WHO/HTM/STB/2006.37. Geneva, Switzerland: WHO; 2006. The Stop TB Strategy. Building and enhancing DOTS to meet the TB-related millennium development goals. [Google Scholar]
- 7.Mohammed A, Ehrlich R, Wood R, Cilliers F, Maartens G. Screening for tuberculosis in adults with advanced HIV infection prior to preventive therapy. Int J Tuberc Lung Dis. 2004;8:792–795. [PubMed] [Google Scholar]
- 8.Programme National Integre de Lutte contre la Lepre et la Tuberculose (PNILT) Manuel technique de la tuberculose. Kigali, Rwanda: Ministry of Health; 2005. [Google Scholar]