Figure 3.
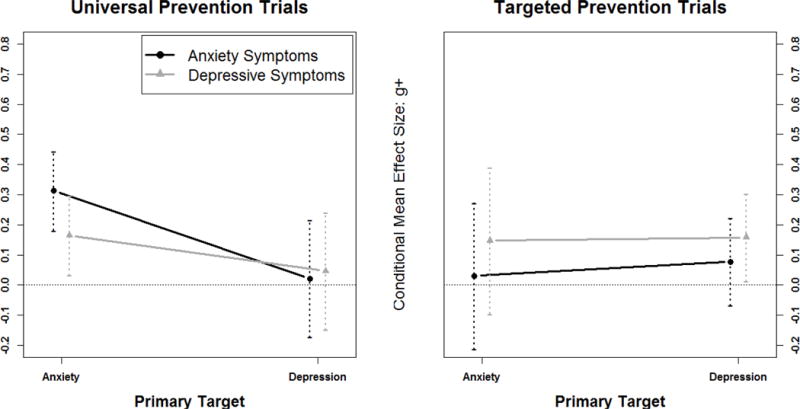
Three-way interaction of outcome*risk*target from the post-hoc analysis. Among prevention trials, the magnitude of intervention effects varied depending on whether trials used universal or targeted participant selection approaches (risk). The pattern of the risk effect differed depending on whether the intervention was primarily targeting anxiety or depression (target) and whether the outcome variable was anxiety or depressive symptoms (outcome). Anxiety prevention programs using universal samples had significant benefits on both anxiety and depressive symptoms (i.e., cross-over effect). Depression prevention programs with targeted (i.e., at risk) samples yielded significant benefits on depressive symptoms, but not anxiety symptoms. There were no benefits on either depressive symptoms or anxiety symptoms among universal depression prevention trials and targeted anxiety prevention trials.
