Abstract
Background
Gallbladder and extrahepatic biliary malignancies are aggressive tumors with high risk of recurrence and death. We hypothesize that elevated preoperative Neutrophil-Lymphocyte Ratios (NLR) are associated with poor prognosis among patients undergoing resection of gallbladder or extrahepatic biliary cancers.
Methods
Patients who underwent complete surgical resection between 2000–2014 were identified from 10 academic centers (n=525). Overall (OS) and recurrence-free survival (RFS) were analyzed by stratifying patients with normal (<5) versus elevated (>5) NLR.
Results
Overall, 375 patients had NLR <5 while 150 patients had NLR >5. Median OS was 24.5 months among patients with NLR<5 versus 17.0 months among patients with NLR>5 (p<0.001). NLR was also associated with OS in subgroup analysis of patients with gallbladder cancer. In fact, on multivariable analysis, NLR>5, dyspnea and preoperative peak bilirubin were independently associated with OS in patients with gallbladder cancer. Median RFS was 26.8 months in patients with NLR<5 versus 22.7 months among patients with NLR>5 (p=0.030). NLR>5 was independently associated with worse RFS for patients with gallbladder cancer.
Conclusions
Elevated NLR was associated with worse outcomes in patients with gallbladder and extrahepatic biliary cancers after curative-intent resection. NLR is easily measured and may provide important prognostic information.
Introduction
Neutrophil-Lymphocyte Ratio (NLR) is associated with poor outcomes in many solid-tumors. Elevated NLR is independently associated with worse outcomes in cancers of the breast,1, 2 thyroid,3 colon,4 stomach,5 prostate,6 lung,7 adrenal,8 pancreas9, 10 urogenital tract,11 esophagus12 and in glioblastoma,13 as well as hematologic cancers, including multiple myeloma14 and diffuse large B-cell lymphoma.15 According to a recent systematic review and meta-analysis including 100 studies and greater than 40,000 patients, high NLR is associated with adverse overall survival (OS) in many solid tumors.16 Another meta-analysis evaluating 49 studies and including 14,282 patients reports elevated NLR is associated with poor OS and disease-free survival (DFS).17 The important role that inflammation plays in cancer and operative outcomes is being increasingly recognized.18, 19, 20, 21, 22 Increased preoperative NLR may be an important indicator of the inflammatory state of patient at the time of surgery. This marker is easily obtained from a patient's routine preoperative laboratory studies and is easily calculated.
However, the role in prognosis of NLR has not been well examined in extrahepatic biliary cancers and studies examining NLR in the liver and pancreas cancer populations to date have been relatively small and some have combined patients undergoing resections for pancreatic and biliary cancers, as well as for hepatic metastasis from non-hepatobiliary primaries. In order to explore the impact of NLR as a biomarker in patients with extrahepatic biliary and gallbladder cancers, we utilized the United States Biliary Malignancy Consortium (US-BMC) database. We hypothesize that elevated NLR is associated with a worse prognosis among patients undergoing curative-intent resection of gallbladder and extrahepatic biliary cancer.
Materials and methods
Study population and data collection
The United States Biliary Malignancy Consortium (US-BMC) is a group of 10 U.S. Academic Medical Centers (The Ohio State University, Columbus, Ohio; Emory University, Atlanta, Georgia; University of Wisconsin, Milwaukee, Wisconsin; Johns Hopkins University, Baltimore, Maryland; Stanford University, Stanford, California; New York University, New York, New York; Washington University, St. Louis, Missouri; Vanderbilt University, Nashville, Tennessee; University of Louisville, Louisville, Kentucky; Wake Forest University, Winston-Salem, North Carolina). The US-BMC compiled a database of 1092 patients with distal or hilar cholangiocarcinoma, or gallbladder cancer who underwent operation between January 1, 2000 and December 31, 2014. Independent manual chart review was performed at each institution and data was entered in a standardized data collection sheet. Inclusion criteria for this study included having undergone curative-intent, complete resection and patients with preoperative complete blood count (CBC) with differential allowing calculation of NLR. Patients using chronic steroids were excluded from analysis due to the interaction with white blood cell count. The institutional review boards of all participating institutions approved the study.
Patient demographics and preoperative comorbidities were manually extracted from the patient's electronic and paper records independently at each institution. Less than 5% of patients had missing data regarding preoperative comorbidities. Tumor size, margin and lymph node status were determined by final pathologic examination. Staging was based on AJCC 7th edition criteria for gallbladder cancer and distal cholangiocarcinoma. NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes based on most immediate preoperative CBC collected within 30 days of operation. CBCs were collected within 30 days and for patients with multiple CBCs collected during this time period the one collected closest to the date of surgery was used. For the purposes of this study NLR greater than or equal to 5 was defined as elevated.8, 17, 23, 24 Postoperative complications were manually extracted from each patient's chart at each institution and entered into the standardized data collection sheet. If no complication was experienced, nothing was noted.
Statistical analysis
Clinicopathologic characteristics were recorded and patient cohorts were analyzed stratified by NLR <5 and NLR ≥5. Categorical variables were analyzed using the Chi-square test or Fisher's exact tests and Wilcoxon test was used for continuous variables. Overall survival (OS) was defined as the time from date of surgery to date of death. Patients who were alive at the date of last observation were censored for survival analysis. Recurrence free survival (RFS) was defined as the time from date of surgery to date of disease recurrence. Patients who were disease free at the date of last observation were censored. Survival curves were estimated using the Kaplan–Meier method log-rank tests stratified according to NLR (between NLR <5 and NLR ≥5). Univariate Cox Regression were fit for each variable first, then multivariable Cox regression models were fit to OS and RFS, respectively using all the variables with p < 0.15 in the univariate analysis. Only variables that were available preoperatively were included in univariate and multivariate analysis. Variables with p > 0.05 were removed sequentially from the Cox regression model using the backward selection method. All statistical analyses were conducted using SAS for Windows® Version 9.2 (SAS Institute Inc., Cary, NC). A p-value <0.05 was considered significant.
Results
There were 525 patients who qualified for inclusion. In assessing the entire cohort, 187 (36%) patients had gallbladder cancer, 189 (36%) had distal cholangiocarcinoma and 149 (28%) had hilar cholangiocarcinoma. There were 375 patients with NLR <5 (71%) and 150 patients with NLR ≥5 (29%). Factors associated with elevated NLR were male sex, age, diagnosis and type of resection (Table 1). Of these diagnosis was independently associated with NLR on multivariable analysis (p = 0.020).
Table 1.
Clinicopathological features and outcomes stratified by NLR
| Characteristic | NLR |
||
|---|---|---|---|
| <5 | ≥5 | P | |
| N | 375 | 150 | |
| Male sex | 180 (48.0) | 86 (57.3) | 0.053 |
| Age, yr, median (IQR) | 67 (57–73) | 69 (61–77) | 0.010 |
| White race | 273 (76.0) | 114 (80.3) | 0.308 |
| ASA | 0.359 | ||
| 1 or 2 | 105 (37.4) | 36 (32.4) | |
| 3 or 4 | 176 (62.6) | 75 (67.6) | |
| Diagnosis | 0.019 | ||
| Gallbladder cancer | 145 (38.7) | 42 (28.0) | |
| Distal cholangiocarcinoma | 122 (32.5) | 67 (44.7) | |
| Hilar cholangiocarcinoma | 108 (28.8) | 41 (27.3) | |
| Margin status | 0.532a | ||
| R0 | 296 (79.6) | 113 (75.8) | |
| R1 | 75 (20.2) | 36 (24.2) | |
| R2 | 1 (0.3) | 0 (0) | |
| AJCC T stage | |||
| Gallbladder | 0.736a | ||
| 0 | 3 (2.2) | 0 (0) | |
| 1 | 7 (5.1) | 2 (4.9) | |
| 2 | 58 (42.0) | 14 (34.2) | |
| 3 | 58 (42.0) | 21 (51.2) | |
| 4 | 9 (6.5) | 2 (4.9) | |
| 5 | 3 (2.2) | 2 (4.9) | |
| Distal cholangiocarcinoma | 0.317a | ||
| 1 | 4 (3.6) | 5 (8.6) | |
| 2 | 34 (30.6) | 13 (22.4) | |
| 3 | 66 (59.5) | 38 (65.5) | |
| 4 | 7 (6.3) | 2 (3.5) | |
| Hilar cholangiocarcinoma | 0.992a | ||
| 0 | 9 (12.2) | 4 (11.4) | |
| 1 | 21 (28.4) | 11 (31.4) | |
| 2 | 28 (37.8) | 14 (40.0) | |
| 3 | 13 (17.6) | 5 (14.3) | |
| 4 | 3 (4.1) | 1 (2.9) | |
| Lymph node positive | 153 (44.5) | 60 (46.9) | 0.642 |
| Type of resection | 0.038 | ||
| Bile duct resection only | 34 (9.1) | 19 (12.7) | |
| Cholecystectomy only | 14 (3.8) | 9 (6.0) | |
| Radical cholecystectomy (Segments IVb+V) + Portal LN dissection | 123 (33.0) | 30 (20.0) | |
| Right hepatectomy + Bile duct resection | 15 (4.0) | 7 (4.7) | |
| Left hepatectomy + Bile duct resection | 31 (8.3) | 12 (8.0) | |
| Extended right hepatectomy + Bile duct resection | 17 (4.6) | 9 (6.0) | |
| Extended left hepatectomy + Bile duct resection | 9 (2.4) | 4 (2.7) | |
| Right trisectorectomy + Bile duct resection | 20 (5.4) | 1 (0.7) | |
| Left trisectorectomy + Bile duct resection | 9 (2.4) | 6 (4.0) | |
| Pylorus-preserving Whipple | 39 (10.5) | 19 (12.7) | |
| Classic whipple | 59 (15.8) | 34 (22.7) | |
| Whipple + Right hepatectomy | 3 (0.80) | 0 (0) | |
| In-hospital mortality | 20 (5.3) | 3 (2.0) | 0.103a |
| Complications | 197 (55.3) | 93 (66.0) | 0.030 |
| LOS, days, median (IQR) | 8 (6–14) | 9 (7–15) | 0.025 |
| Reoperation | 24 (6.5) | 11 (7.5) | 0.673 |
| Neoadjuvant chemotherapy | 10 (2.7) | 4 (2.7) | 1a |
| Adjuvant chemotherapy | 175 (54.2) | 63 (49.6) | 0.382 |
P-values in bold in Table 1 indicate statistical significance with p<0.05.
Fisher's exact test.
Patients with NLR <5 and NLR ≥5 had similar rates of in-hospital mortality (5.3% versus 2.0%, p = 0.103). Patients with NLR <5 experienced less complications than patients with NLR ≥5 (55.3% versus 66.0%, p = 0.030). Patients with NLR <5 had shorter lengths of stay (median 8 days versus 9 days, p = 0.025) but experienced similar rates of reoperation (6.5% versus 7.5%, p = 0.673) as patients with NLR ≥5. Few patients in either group received neoadjuvant chemotherapy (2.7% and 2.7%), and a similar proportion in each group received adjuvant chemotherapy (54.2% versus 49.6%, p = 0.382).
Commonly performed procedures included radical cholecystectomy and portal lymph node dissection for patients with gallbladder cancer, standard pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy for patients with distal cholangiocarcinoma and isolated bile duct resection. Additionally, many patients with hilar cholangiocarcinoma underwent bile duct resection with left or right, or extended left or right, hepatectomy.
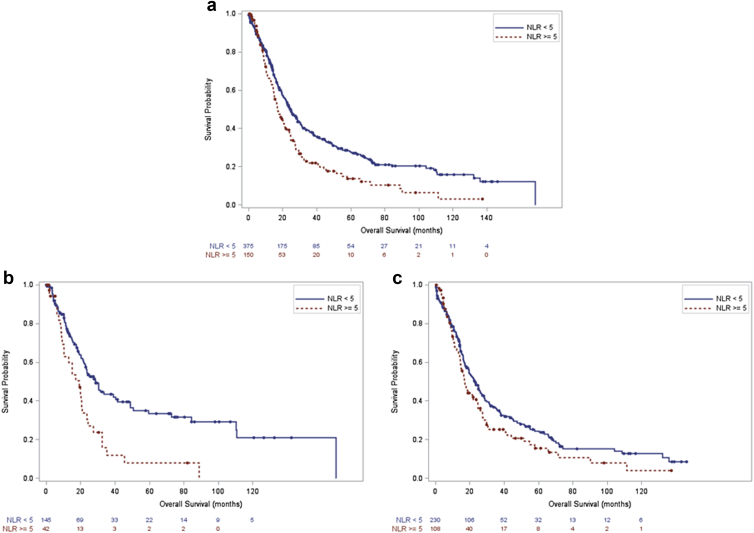
In the entire cohort (Fig. 1a, log-rank test, p < 0.001) and among the subgroup of patients with gallbladder cancer (Fig. 1b, log-rank test, p < 0.001), overall survival (OS) was higher in patients with NLR <5 than patients with NLR ≥5. In the subgroup of patients with extrahepatic cholangiocarcinoma there was a similar trend, but this did not reach statistical significance (Fig. 1c, log-rank test, p = 0.068). In the entire cohort the 1-, 3- and 5-year survival were 75%, 38% and 28% in the group with NLR <5 versus 66%, 22% and 14% in the group with NLR ≥5. Among patients with gallbladder cancer 1-, 3- and 5-year survival were 76%, 43% and 34% among patients with NLR <5 versus 63%, 12% and 8% among patients with NLR ≥5. In patients with extrahepatic cholangiocarcinoma the 1-, 3- and 5-year survival were 75%, 35% and 24% in the group with NLR <5 versus 67%, 25% and 16% in the group with NLR ≥5. NLR ≥5 was independently associated with worse OS in patients with gallbladder cancer (Table 2, HR 3.52, 95% CI 1.58–7.85), but was not associated by multivariate analysis with OS in the entire cohort or in patients with extrahepatic cholangiocarcinoma (data not shown).
Figure 1.
a. Overall survival NLR <5 versus NLR ≥5, entire cohort, (p < 0.001). b. Overall survival NLR <5 versus NLR ≥5, gallbladder cancer, (p < 0.001). c. Overall survival NLR <5 versus NLR ≥5, extrahepatic cholangiocarcinoma (p = 0.068)
Table 2.
Predictive factors for overall survival in gallbladder cancer
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| p-value | Hazard ratio | p-value | Hazard ratio | |
| Neutrophil-lymphocyte ratio ≥5 | <0.001 | 2.08 [1.35, 3.21] | 0.002 | 3.52 [1.58, 7.85] |
| Dyspnea | 0.05 | 2.32 [1.00, 5.37] | 0.009 | 4.48 [1.45, 14.77] |
| Severe COPD | 0.08 | 2.44 {0.88, 6.73] | ||
| Age | 0.09 | 1.02 [1.00, 1.03] | ||
| White blood cell count | 0.07 | 1.04 [1.00, 1.09] | ||
| Preoperative peak bilirubin | <0.001 | 1.09 [1.05, 1.13] | <0.001 | 1.12 [1.05, 1.20] |
| Last bilirubin | <0.001 | 1.10 [1.03, 1.19] | ||
| Albumin | <0.001 | 0.56 [0.42, 0.74] | ||
| INR | 0.01 | 3.09 [1.33, 7.19] | ||
| CA 19-9 | <0.001 | 1.00 [1.00, 1.00] | ||
| Platelet-lymphocyte ratio | 0.06 | 0.99 [0.97, 1.00] | ||
Variables, p-values and hazard ratios in bold indicate those which were found to be significant on multivariate analysis.
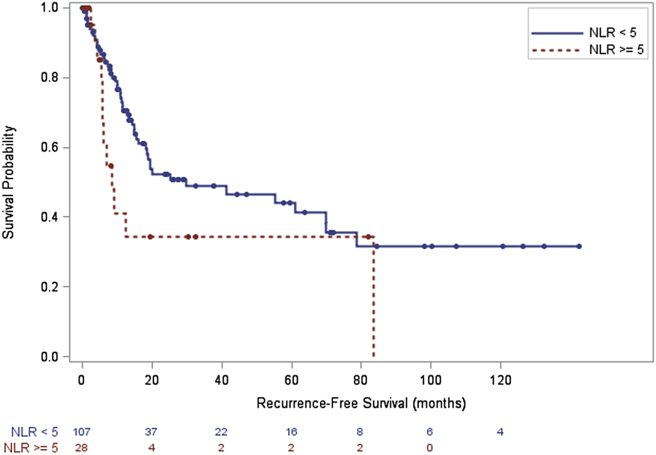
In the entire cohort (Fig. 2, log-rank test, p = 0.030) recurrence-free survival (RFS) was significantly higher in patients with NLR <5 in comparison to patients with NLR ≥5. Among the subgroup of patients with gallbladder cancer there was a similar trend, but this did not reach statistical significance (log-rank test, p = 0.084). In the entire cohort median 1-, 3- and 5-year RFS were 76%, 44% and 39% in the group with NLR <5 versus 65%, 34% and 27% in the group with NLR ≥5. In the subgroup with gallbladder cancer median, 1-, 3- and 5-year RFS were 70%, 49% and 44% in the group with NLR <5 versus 41%, 34% and 34% in the group with NLR ≥5. There was also not a significant difference in RFS based on NLR cutoff of 5 in patients with extrahepatic cholangiocarcinoma. NLR ≥5 was independently associated with worse RFS for patients with gallbladder cancer (Table 3, HR 4.63 95% CI 1.48–14.51), but was not associated on multivariate analysis with RFS in the entire cohort or patients with extrahepatic cholangiocarcinoma.
Figure 2.
Recurrence free survival NLR <5 versus NLR ≥5, entire cohort, (p = 0.030)
Table 3.
Predictive factors for recurrence-free survival in gallbladder cancer
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| p-value | Hazard ratio | p-value | Hazard ratio | |
| Neutrophil-lymphocyte ratio ≥5 | 0.09 | 1.75 [0.92, 3.31] | 0.009 | 4.63 [1.48, 14.51] |
| Hypertension | 0.15 | 1.50 [0.86, 2.60] | ||
| Diabetes – insulin dependent | 0.10 | 2.41 [0.85, 6.80] | <0.001 | 432 [15.6, 12024.4] |
| Dyspnea | 0.06 | 3.29 [0.92, 15.89] | ||
| Severe COPD | 0.06 | 2.13 [0.95, 10.26] | ||
| Systemic sepsis | 0.02 | 5.59 [1.32, 23.55] | 0.005 | 70.6 [3.4, 1430.8] |
| Ascites | 0.02 | 11.72 [1.49, 92.51] | ||
| White blood cell count | 0.09 | 1.08 [0.99, 1.17] | ||
| Peak bilirubin | <0.001 | 1.09 [1.03, 1.15] | <0.001 | 1.16 [1.06, 1.26] |
| Last bilirubin | 0.03 | 1.12 [1.01, 1.23] | ||
| Albumin | 0.11 | 0.70 [0.45, 1.09] | ||
| INR | 0.09 | 2.54 [0.89, 7.30] | ||
| CA 19-9 | 0.02 | 1.00 [1.00, 1.00] | ||
Variables, p-values and hazard ratios in bold indicate those which were found to be significant on multivariate analysis.
Discussion
The present study of nearly 700 patients with gallbladder and extrahepatic biliary cancers demonstrate that elevated NLR is associated with worse outcomes after curative-intent surgical resections. This finding is consistent with other smaller studies demonstrating that elevated NLR portends poor prognosis in solid tumor malignancies.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
Other studies in patients with hepato-pancreato-biliary (HPB) cancers agree with the findings presented in this paper. In a study of 452 patients who underwent a HPB procedure for malignant disease, NLR >5 was found to be associated with worse OS.23 Elevated NLR is associated with worse survival after hepatectomy for intrahepatic cholangiocarcinoma.24, 25 Elevated preoperative NLR is associated with worse OS in gallbladder cancer at a lower threshold (>1.94) than the current study according to at least one other group of investigators.26
Despite the robust and growing data regarding the utility of the NLR, findings are not uniform across all publications. For patients with gastric cancer, the Glasgow prognostic score and Tumor Node Metastasis (TNM) staging system may be more robust predictors of survival than NLR.27 Studies are not concordant for whether elevated NLR is28 or is not29 associated with worse outcomes for patients with cholangiocarcinoma. It is therefore perhaps noteworthy that elevated NLR was not as strongly associated with cholangiocarcinoma outcomes in this study as it was for patients with gallbladder cancer.
Our group has previously published the importance of NLR trend comparing values before and after therapy, specifically before and after chemoembolization in patients with hepatocellular carcinoma. In that study, patients whose NLR rose 1 month after TACE or remained elevated had significantly worse survival than those whose NLR normalized or remained normal.30 NLR trend may be another potentially useful biomarker for HPB and other cancers. Other potential uses of NLR besides survival outcomes may include identifying patients who might benefit from adjuvant therapy.17 Stratifying patients based on pretreatment NLR within treatment arms in clinical trials may provide additional information regarding response to therapy and may aid in personalization of treatment. NLR has been used in other cancer types to identify patients least likely to respond to chemotherapy.31 The mechanism behind the association of NLR and worse prognosis in these and other cancers has not been established. This is an important arena for further studies.
There are limitations that should be considered in the interpretation of this study. The data described are derived from a multi-institutional cohort and therefore there was no standardization of operative procedures or perioperative approach between centers. A limitation of the collaborative in general is the combination of three types of cancer. Additionally, a substantial number of patients were excluded due to missing neutrophil and lymphocyte data. The advantages of using this multi-institutional cohort include achievement of an improved sample size, increased generalizability of the results and reduction of potential biases observed in single-institution observational studies. Although the sample size achieved was improved over that which could be obtained at a single institution, a larger sample size may desirable.
Gallbladder and extrahepatic biliary malignancies are aggressive cancers with high rates of recurrence and death even after surgical resection. Neutrophil-lymphocyte ratio is a readily available biomarker that can be calculated without obtaining additional costly laboratory testing. Thus, its' value should be calculated by oncologists of all disciplines and incorporated with other prognostic information. Further, it would be reasonable to stratify patients for clinical trials based on pre-therapy NLR.
Sources of funding
There was no funding associated with the completion of this work.
Conflicts of interest
None declared.
Footnotes
This paper was presented at the IHPBA World Congress, April 20–23, 2016 in Sao Paulo, Brazil.
References
- 1.Koh C.H., Bhoo-Pathy N., Ng K.L., Jabir R.S., Tan G.H., See M.H. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015 Jun;113:150–158. doi: 10.1038/bjc.2015.183. PubMed PMID: 26022929. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krenn-Pilko S., Langsenlehner U., Stojakovic T., Pichler M., Gerger A., Kapp K.S. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2015 Jul;37:361–368. doi: 10.1007/s13277-015-3805-4. PubMed PMID: 26219894. ENG. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.Y., Park T., Jeong S.H., Jeong C.Y., Ju Y.T., Lee Y.J. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014 Aug;46:526–531. doi: 10.1007/s12020-013-0089-6. PubMed PMID: 24272600. eng. [DOI] [PubMed] [Google Scholar]
- 4.Shin J.S., Suh K.W., Oh S.Y. Preoperative neutrophil to lymphocyte ratio predicts survival in patients with T1-2N0 colorectal cancer. J Surg Oncol. 2015 Oct;112:654–657. doi: 10.1002/jso.24061. PubMed PMID: 26437893. ENG. [DOI] [PubMed] [Google Scholar]
- 5.Lian L., Xia Y.Y., Zhou C., Shen X.M., Li X.L., Han S.G. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015 Sep;15:899–907. doi: 10.3233/CBM-150534. PubMed PMID: 26444485. ENG. [DOI] [PubMed] [Google Scholar]
- 6.Lee H., Jeong S.J., Hong S.K., Byun S.S., Lee S.E., Oh J.J. High preoperative neutrophil-lymphocyte ratio predicts biochemical recurrence in patients with localized prostate cancer after radical prostatectomy. World J Urol. 2015 Oct;34:821–827. doi: 10.1007/s00345-015-1701-6. PubMed PMID: 26449784. ENG. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K., Okita R., Saisho S., Maeda A., Nojima Y., Nakata M. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol. 2015;13:291. doi: 10.1186/s12957-015-0710-7. PubMed PMID: 26424708. PMCID: PMC4590710. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagante F., Tran T.B., Postlewait L.M., Maithel S.K., Wang T.S., Evans D.B. Neutrophil-lymphocyte and platelet-lymphocyte ratio as predictors of disease specific survival after resection of adrenocortical carcinoma. J Surg Oncol. 2015 Aug;112:164–172. doi: 10.1002/jso.23982. PubMed PMID: 26234285. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh B.K., Tan D.M., Chan C.Y., Lee S.Y., Lee V.T., Thng C.H. Are preoperative blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios useful in predicting malignancy in surgically-treated mucin-producing pancreatic cystic neoplasms? J Surg Oncol. 2015 Aug;112:366–371. doi: 10.1002/jso.23997. PubMed PMID: 26280242. eng. [DOI] [PubMed] [Google Scholar]
- 10.Arima K., Okabe H., Hashimoto D., Chikamoto A., Kuroki H., Taki K. The neutrophil-to-lymphocyte ratio predicts malignant potential in intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2015 Oct;19:2171–2177. doi: 10.1007/s11605-015-2973-2. PubMed PMID: 26443528. ENG. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y., She D.L., Xiong H., Fu S.J., Yang L. Pretreatment neutrophil to lymphocyte ratio as a prognostic predictor of urologic tumors: a systematic review and meta-analysis. Med Baltim. 2015 Oct;94:e1670. doi: 10.1097/MD.0000000000001670. PubMed PMID: 26448011. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yodying H., Matsuda A., Miyashita M., Matsumoto S., Sakurazawa N., Yamada M. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015 Sep;23:646–654. doi: 10.1245/s10434-015-4869-5. PubMed PMID: 26416715. ENG. [DOI] [PubMed] [Google Scholar]
- 13.McNamara M.G., Lwin Z., Jiang H., Templeton A.J., Zadeh G., Bernstein M. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014 Mar;117:147–152. doi: 10.1007/s11060-014-1366-9. PubMed PMID: 24469854. eng. [DOI] [PubMed] [Google Scholar]
- 14.Kelkitli E., Atay H., Cilingir F., Güler N., Terzi Y., Ozatlı D. Predicting survival for multiple myeloma patients using baseline neutrophil/lymphocyte ratio. Ann Hematol. 2014 May;93:841–846. doi: 10.1007/s00277-013-1978-8. PubMed PMID: 24337486. eng. [DOI] [PubMed] [Google Scholar]
- 15.Porrata L.F., Ristow K., Habermann T., Inwards D.J., Micallef I.N., Markovic S.N. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol. 2010 Nov;85:896–899. doi: 10.1002/ajh.21849. PubMed PMID: 20842639. eng. [DOI] [PubMed] [Google Scholar]
- 16.Templeton A.J., McNamara M.G., Šeruga B., Vera-Badillo F.E., Aneja P., Ocaña A. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014 Jun;106 doi: 10.1093/jnci/dju124. dju124. PubMed PMID: 24875653. eng. [DOI] [PubMed] [Google Scholar]
- 17.Paramanathan A., Saxena A., Morris D.L. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014 Mar;23:31–39. doi: 10.1016/j.suronc.2013.12.001. PubMed PMID: 24378193. eng. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001 Feb;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. PubMed PMID: 11229684. eng. [DOI] [PubMed] [Google Scholar]
- 19.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012 Dec;125(Pt 23):5591–5596. doi: 10.1242/jcs.116392. PubMed PMID: 23420197. eng. [DOI] [PubMed] [Google Scholar]
- 20.Wortel C.H., van Deventer S.J., Aarden L.A., Lygidakis N.J., Büller H.R., Hoek F.J. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery. 1993 Sep;114:564–570. PubMed PMID: 7690162. eng. [PubMed] [Google Scholar]
- 21.Beilin B., Bessler H., Mayburd E., Smirnov G., Dekel A., Yardeni I. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. 2003 Jan;98:151–155. doi: 10.1097/00000542-200301000-00024. PubMed PMID: 12502991. eng. [DOI] [PubMed] [Google Scholar]
- 22.Shavit Y., Fridel K., Beilin B. Postoperative pain management and proinflammatory cytokines: animal and human studies. J Neuroimmune Pharmacol. 2006 Dec;1:443–451. doi: 10.1007/s11481-006-9043-1. PubMed PMID: 18040817. eng. [DOI] [PubMed] [Google Scholar]
- 23.Spolverato G., Maqsood H., Kim Y., Margonis G., Luo T., Ejaz A. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015 Jun;111:868–874. doi: 10.1002/jso.23900. PubMed PMID: 25865111. eng. [DOI] [PubMed] [Google Scholar]
- 24.Gomez D., Morris-Stiff G., Toogood G.J., Lodge J.P., Prasad K.R. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008 May;97:513–518. doi: 10.1002/jso.21001. PubMed PMID: 18335453. eng. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Yang L.X., Li X.D., Yin D., Shi S.M., Chen E.B. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumour Biol. 2015 Jul;36:5283–5289. doi: 10.1007/s13277-015-3188-6. PubMed PMID: 25672606. eng. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Jiang C., Li J., Sun J., Qu X. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clin Transl Oncol. 2015 Oct;17:810–818. doi: 10.1007/s12094-015-1310-2. PubMed PMID: 26077119. eng. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q.X., Su Z.J., Zhang J.H., Wang C.R., Ke S.Y. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther. 2015;8:1375–1385. doi: 10.2147/OTT.S82437. PubMed PMID: 26124667. PMCID: PMC4476486. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNamara M.G., Templeton A.J., Maganti M., Walter T., Horgan A.M., McKeever L. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014 Jun;50:1581–1589. doi: 10.1016/j.ejca.2014.02.015. PubMed PMID: 24630393. eng. [DOI] [PubMed] [Google Scholar]
- 29.Hakeem A.R., Marangoni G., Chapman S.J., Young R.S., Nair A., Hidalgo E.L. Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil-lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma? Eur J Gastroenterol Hepatol. 2014 Sep;26:1047–1054. doi: 10.1097/MEG.0000000000000162. PubMed PMID: 25051217. eng. [DOI] [PubMed] [Google Scholar]
- 30.McNally M.E., Martinez A., Khabiri H., Guy G., Michaels A.J., Hanje J. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol. 2013 Mar;20:923–928. doi: 10.1245/s10434-012-2639-1. PubMed PMID: 22965570. eng. [DOI] [PubMed] [Google Scholar]
- 31.Chua W., Charles K.A., Baracos V.E., Clarke S.J. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011 Apr;104:1288–1295. doi: 10.1038/bjc.2011.100. PubMed PMID: 21448173. PMCID: PMC3078587. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]