Abstract
Innate-like B lymphocytes play an important role in innate immunity in periodontal disease through Toll-like receptor (TLR) signaling. However, it is unknown how innate-like B cell apoptosis is affected by the periodontal infection-associated innate signals. This study is to determine the effects of two major TLR ligands, lipopolysaccharide (LPS) and CpG-oligodeoxynucleotides (CpG-ODN), on innate-like B cell apoptosis. Spleen B cells were isolated from wild type (WT), TLR2 knockout (KO) and TLR4 KO mice and cultured with E. coli LPS alone, P. gingivalis LPS alone, or combined with CpG-ODN for 2 days. B cell apoptosis and expressions of specific apoptosis-related genes were analyzed by flow cytometry and real-time PCR respectively. P. gingivalis LPS, but not E. coli LPS, reduced the percentage of AnnexinV+/7-AAD- cells within IgMhighCD23lowCD43-CD93- marginal zone (MZ) B cell sub-population and IgMhighCD23lowCD43+CD93+ innate response activator (IRA) B cell sub-population in WT but not TLR2KO or TLR4KO mice. CpG-ODN combined with P. gingivalis LPS further reduced the percentage of AnnexinV+/7-AAD- cells within MZ B cells and IRA B cells in WT but not TLR2 KO or TLR4 KO mice. Pro-apoptotic CASP4, CASP9 and Dapk1 were significantly down-regulated in P. gingivalis LPS- and CpG-ODN-treated B cells from WT but not TLR2 KO or TLR4 KO mice. Anti-apoptotic IL-10 was significantly up-regulated in P. gingivalis LPS- and CpG-ODN-treated B cells from WT and TLR2 KO but not TLR4 KO mice. These results suggested that both TLR2 and TLR4 signaling are required for P. gingivalis LPS-induced, CpG-ODN-enhanced suppression of innate-like B cell apoptosis.
Introduction
Innate immune system recognizes pathogen-associated molecular patterns with a set of germline-encoded pattern-recognition receptors including Toll-like receptors (TLRs) [1, 2]. TLRs play important roles in the process of B cell proliferation and apoptosis, and studies have shown that TLR2, TLR4 and TLR9 are all expressed in murine B cells [3, 4] as well as in human B cells [5, 6]. As multiple TLRs could be activated simultaneously by their corresponding ligands during immune response to pathogens in diseases, the effect of co-activation of these TLR pathways on B cell apoptosis has not been investigated.
Periodontal disease is an infection-associated, immune-mediated oral disease leading to the gingival tissue destruction [7], alveolar bone resorption [8], and increased risk of systemic complications [9]. Porphyromonas gingivalis (P. gingivalis), an anaerobic bacterium, is considered one of the principal pathogens of adult periodontitis that can orchestrate inflammatory disease by remodeling a normally benign microbiota into a dysbiotic one [10]. Different from E. coli LPS, which is a definitive TLR4 ligand, P. gingivalis LPS has been shown to be able to activate both TLR2 and TLR4 [11, 12]. Together with the ligation between bacterial DNA component CpG oligodeoxynucleotides (CpG-ODN) and its receptor TLR9 during P. gingivalis infection, it is valuable to determine the effects of multiple TLR activation (TLR2, TLR4 and TLR9) in the regulation of immune B cell functions in order to understand the role of TLR signaling in infection-associated periodontal pathogenesis.
B cells are linked developmentally, reside in different regions in the lymphoid organs, and mediate distinct functions [13]. In mice, three major B subsets have been identified as follicular B2 cells, B1 cells (including CD51B1a and CD52 B1b cells) and marginal zone (MZ) B cells. Innate-like B cells are heterogeneous populations that can rapidly acquire immune regulatory activities through the secretion of natural IgM and IL-10 [14]. These unconventional B cells with autoreactive properties can provide a rapid T cell-independent antibody response to protect against infections [15]. Innate-like B cells in mice are composed of B1 cells [16], marginal zone (MZ) B cells [17] and other related B cells [18]. Recent studies indicated that innate-like B cells can link innate immunity to adaptive immune responses during infection [19, 20].
Programmed cell death, including apoptosis, autophagy and programmed necrosis, is mediated by intracellular programs to decide the fate of cells [21]. Among the three forms of programmed cell death, apoptosis is a major event during immune cell development and responses to extracellular stimuli. Regulation of immune cell apoptosis is essential for the maintenance of immune system homeostasis [22, 23], and dysregulation of apoptosis in B cells may cause autoimmune manifestations [24]. Although numerous studies have indicated the key role of TLR signaling in the regulation of non-immune cell apoptosis [25, 26], the potential role of multiple TLRs in the control of innate-like B cell apoptosis is completely unknown.
The purpose of the study is to evaluate the role of specific TLRs on the innate-like B cell apoptosis using periodontal pathogen-associated TLR ligands (P. gingivalis LPS and CpG-ODN). Information on the TLR-mediated control of innate-like B cell apoptosis will give a new insight of host-pathogen interactions in the development of host immune response and periodontal disease pathogenesis.
Materials and Methods
Animals
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). TLR2 knockout (KO) and TLR4 KO mice backcrossed to the C57BL/6 background were a kind gift from Dr. Toshihisa Kawai (Forsyth Institute, Cambridge, USA). All the mice used in the study were from 8 to 10 weeks old and were maintained under pathogen-free conditions in laminar flow cabinets. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Forsyth Institute.
B cell isolation and culture
Mice were euthanized in CO2 chamber and spleens were harvested. All cell culture disposable ware, including tips, tubes, serological pipettes, flasks and culture plates, were purchased from USA Scientific, Inc and were RNase, DNase, DNA, and pyrogen free. Designated biological safety cabinet and work area were used for culture experiments and were stringently cleaned and disinfected at all times. To monitor potential LPS contamination, the presence of bacterial endotoxins in buffers and culture medium were routinely performed by limulus amebocyte lysate (LAL) test using chromogenic endotoxin quantitation kit (Thermo Scientific). Splenic cell suspensions were prepared in MACS buffer (PBS/2mM EDTA/0.5% BSA). Non-B cells were depleted by incubating splenic cell suspensions with biotin-conjugated antibodies against CD4, CD11c, CD49b, CD90, Gr-1, and Ter119, followed by incubation with anti-biotin antibodies coupled magnetic beads (Miltenyi Biotec). Unlabeled cells were collected by magnetic depletion of labeled cells (contained >98.5% CD19+ cells). Isolated B cells were adjusted to 1×106/ml and were added into either 96-well plates (200μl/well) in IMDM complete medium containing 10% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM L-glutamine, 2.5μg/ml Amphotericin B (Hyclone, Thermo Fisher Scientific, IL) and 50 μM 2-ME. Cells were cultured at 37°C in a humidified incubator with 5% CO2. The TLR ligands were added to the B cells culture as follows: E. coli LPS (10μg/ml, strain O55:B5, Sigma-Aldrich), P. gingivalis LPS (10μg/ml, strain ATCC 33277, InvivoGen) and mouse stimulatory CpG-ODN (10μM, 5’-TCGTCGTTTTGTCGTTTTGTCGTT-3’, Hycult Biotech).
B cell proliferation analysis
B cells (2×105/well) were cultured in 200μl complete medium in 96-well plate for 2 days in the presence of E. coli LPS (10μg/ml), P. gingivalis LPS (10μg/ml), E. coli LPS (10μg/ml) + CpG (10μM), or P. gingivalis LPS (10μg/ml) + CpG (10μM). To determine the number of viable cells in proliferation, MTS reagent was added (40μl/well) 4 hours before the termination of the experiment using a CellTiter 96 AQueous Assay kit (Promega Corp). After 4 hour incubation, the plate was read at OD 490nm using a microplate reader (BioTek). The absorbance of the formazan at 490nm was measured as an indication of cell proliferation. Cell proliferation was also measured by CellTrace Cell Proliferation kit (Invitrogen) following manufacture instructions. Briefly, cells were stained with CellTrace CFSE reagents for 20 minutes and then incubated in culture medium for 10 minutes to undergo acetate hydrolysis. Proliferated cell were analyzed by flow cytometry at 488 nm excitation wavelength and at least 20,000 cells were counted for each sample.
B cell apoptosis analysis by flow cytometry
Isolated B cells (1×106/well) in 200μl culture medium were cultured for 48 hours in “U” bottom 96-well plate with E. coli LPS (10μg/ml), P. gingivalis LPS (10μg/ml), E. coli LPS (10μg/ml) + CpG (10μM), or P. gingivalis LPS (10μg/ml) + CpG (10μM). At the termination of cell culture, B cells in the 96-well plates were washed with PBS followed by incubation with fluorescence conjugated antibodies. The following anti-mouse monoclonal antibodies (mAbs) were used in subpopulation analysis to distinguish cells: PE-conjugated anti IgM, PerCP-Cy5.5-conjugated anti CD23, APC-conjugated anti CD93, Pacific Blue-conjugated anti CD43 (BD Biosciences). The following mAbs were also used for the analysis of B cell apoptosis: FITC- or PE-conjugated Annexin V (BD), 7-Aminoactinomycin D (7-AAD) (BioLegend). Annexin V+7-AAD- cells are considered as early apoptotic cells and Annexin V+7-AAD+ cells are considered as late apoptotic cells. At least 50,000 cells were counted for each sample.
Apoptosis-related gene array
Total RNA was extracted from cultured B cells using a Purelink RNA mini kit (Invitrogen). The mouse RT2 ProfilerPCR array for Apoptosis (PAMM-012Z, SA Biosciences) were used to profile expression of 84 apoptosis-related genes involved in programmed cell death, using a Roche real-time PCR machine (Roche Diagnostics Corporation, Indianapolis, IN). The data for biological duplicates were analyzed using the PCR Array Data Analysis Software (SABiosciences).
Real-time PCR
Total RNA was extracted from the cultured B cells using a Purelink RNA mini kit (Life Technology, Carlsbad, CA) following manufacturer’s instructions. Isolated mRNA (0.1μg each) was reverse transcribed into cDNA using the SuperScriptII reverse transcription system in the presence of random primers (Invitrogen). The real-time PCR was carried out in a 20μl reaction system using SuperScript II Platinum SYBR Green Two-Step qRT-PCR Kit (Life Technology) in a Roche LightCycler 480 (Roche Diagnostics, Indianapolis, IN). Each cDNA sample was loaded in duplicate into the plate with a template amount of 10ng. The primers used for specific genes analyzed were from RT² qPCR Primer Assays (SA Biosciences). The real-time PCR conditions were: 95°C for 10 minutes, followed by 40 cycles of 95°C for 10seconds, 65°C for 10 seconds and 72°C for 15 seconds. Results were presented as fold changes relative to GAPDH reference.
Casp4 and Casp9 activity assay
Splenocyte B cells were separated from WT, TLR2KO and TLR4KO mice and cultured 48 hours with P. gingivalis LPS (10μg/ml), P. gingivalis LPS (10μg/ml) + CpG (10μM) and untreated control. Casp4 and Casp9 protein activities were performed by using Caspase 4 Assay kit (Abcam) or Caspase 9 Assay kit (Abcam) following user’s instruction. Briefly, cells (1×106 per sample) were lysis in 50 μl cell lysis buffer incubated on ice for 10 minutes and then incubated with 50 μl reaction buffer and 5 μl LEHD-AFC substrate at 37°C for 2 hours. The plate was read in a microplate fluorometer reader (BioTek) and fold-increase in Caspase 4/9 activity was determined by comparing these results with the level of the untreated control.
Statistics
Results are presented as means ± standard errors (SE). Paired Student’s t-test was used to analyze differences between two treatments. One-Way ANOVA was used to analyze differences among groups. Results with probability values of less than 0.05 are considered statistically significant.
Results
B cell proliferation after treatment with LPS and CpG-ODN
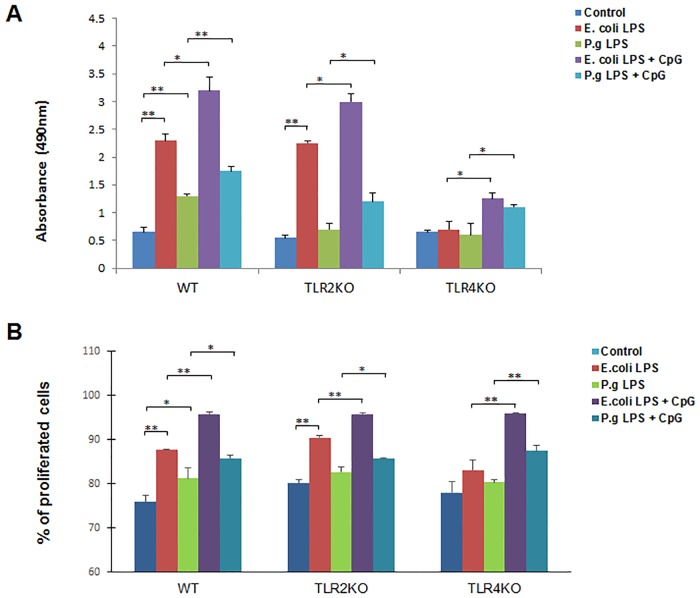
To test the innate proliferative property of B cells in response to the TLRs stimulation, purified B cells were cultured under 5 different conditions (untreated control, E. coli LPS, P. gingivalis LPS, E. coli LPS + CpG-ODN and P. gingivalis LPS + CpG-ODN) and cell proliferation assays were performed after 48 hours. E. coli LPS strongly stimulated the proliferation of B cells from WT and TLR2 KO mice (Fig 1A, 2nd bar in each type of animal). P. gingivalis LPS stimulated proliferation of B cells from WT mice only (Fig 1A, 3rd bar in each type of animal), and the intensity of such stimulation was weaker than those observed in E. coli LPS. In all types of mice, the addition of CpG-ODN together with LPS significantly elevated the proliferation of B cells as compared to those treated with LPS alone (Fig 1A, 4th and 5th bars in each group of animal). To confirm the MTS proliferation results, cell proliferations of each groups were also measured by CellTrace CSFE cell proliferation assay and similar results were observed, demonstrating that the addition of CpG-ODN together with LPS significantly elevated the proliferation of B cells as compared to those treated with LPS alone (Fig 1B).
Fig 1. B cell proliferation after E. coli LPS, P. gingivalis LPS and CpG-ODN treatment.
Splenocyte B cells were separated from WT, TLR2 KO and TLR4 KO mice and cultured 48 hours with E. coli LPS (10μg/ml), P. gingivalis LPS (10μg/ml), E. coli LPS (10μg/ml) + CpG (10μM), and P. gingivalis LPS (10μg/ml) + CpG (10μM). Viable cells quantities were measured by absorbance at 490 nm reading from each group of WT, TLR2 KO and TLR4 KO mice respectively (A) (mean±SE, n = 6, *p<0.05, **p<0.01). Proliferation cells quantities were also measured by CellTrace CSFE staining and presented as percentages of total cells (B). (mean±SE, n = 3, *p<0.05, **p<0.01).
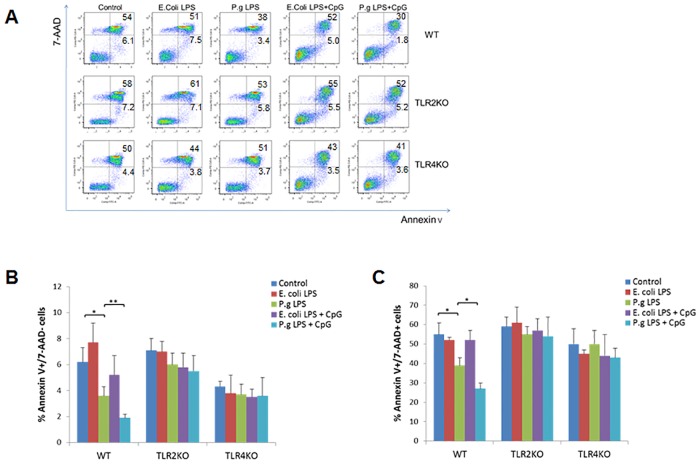
Inhibition of B cell early- and late-apoptosis by P. gingivalis LPS and CpG-ODN
To determine the overall B cell apoptosis, purified B cells from WT and TLRs KO mice were cultured for 2 days under different treatment conditions followed by staining with AnnexinV and 7-AAD and analyzed by flow cytometry (Fig 2A). In WT mice, the percentage of AnnexinV+/7-AAD- (early apoptotic) B cells was significantly decreased after treatment with P. gingivalis LPS (p<0.05) as compared to control group (Fig 2B). The percentage of AnnexinV+/7-AAD- B cells was further reduced when treated with P. gingivalis LPS and CpG-ODN (p<0.01) as compared to group treated with P. gingivalis LPS alone (Fig 2B). However, the percentage of AnnexinV+/7-AAD- B cells was not changed after treatment with E. coli LPS alone, or combined with CpG-ODN, when compared to their respective controls. No changes were observed in the percentage of AnnexinV+/7-AAD- B cells from TLR2 KO or TLR4 KO mice under each treatment condition (Fig 2B). Similar results were observed when the percentage of AnnexinV+/7-AAD+ (late apoptotic/necrotic) B cells was evaluated after different treatments. Only in WT mice, the percentage of AnnexinV+/7-AAD+ B cells was significantly decreased after treatment with P. gingivalis LPS (p<0.05), but not with E. coli LPS, and such effect was further enhanced by the addition of CpG-ODN (p<0.05) (Fig 2C).
Fig 2. B cell early apoptosis and late apoptosis after E. coli LPS, P. gingivalis LPS and CpG-ODN treatment.
Splenocyte B cells were separated from WT, TLR2 KO and TLR4 KO mice and cultured 48 hours with E. coli LPS (10μg/ml), P. gingivalis LPS (10μg/ml), E. coli LPS (10μg/ml) + CpG (10μM), and P. gingivalis LPS (10μg/ml) + CpG (10μM). Cells were then stained by FITC-conjugated AnnexinV mAb and 7-AAD and measured by flow cytometry (A). Percentage of Annexin V+7-AAD- cells (B) and Annexin V+7-AAD+ cells (C) in different treatment groups of WT, TLR2KO and TLR4KO mice were analyzed and compared respectively (mean±SE, n = 6, *p<0.05, **p<0.01).
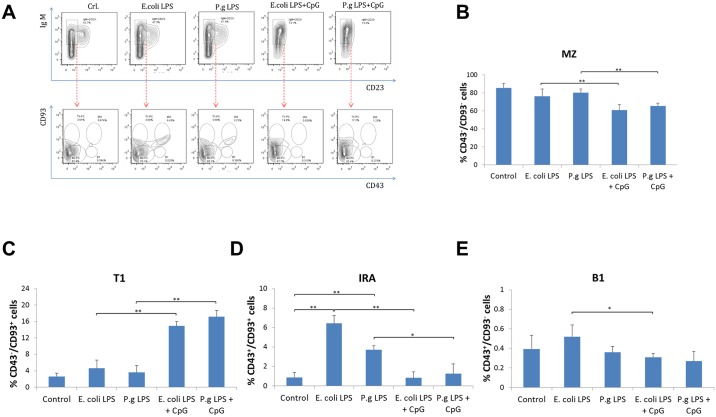
Different responses of innate-like B cell subsets after treatment with LPS and CpG-ODN
The percentage of four sub-types of innate-like B cells in WT mice were detected by flow cytometry using surface markers as previously described [18] to evaluate the innate-like B cell responses to LPS and CpG-ODN stimulation. IgMhighCD23low B cells were selected to represent overall innate-like B cell population based on the previous reports [19, 27], from which the four sub-types of innate-like B cells were identified by CD43 and CD93 labeling (Fig 3A). CD43 has been identified as a marker to define adaptive regulatory B cells from spleen MZ over their innate counterparts of B1 B cells in immune responses against bacterial infection [28]. CD93 has been used to discriminate between transitional 1 (T1) cells and mature B cells [29]. Addition of CpG-ODN to E. coli LPS or P. gingivalis LPS significantly reduced the percentage of CD43-CD93- marginal zone (MZ) B cells (Fig 3B). Contrarily, the percentage of CD43-CD93+ transitional (T1) B cells was largely increased when treated with E. coli LPS or P. gingivalis LPS together with CpG-ODN (Fig 3C). The percentage of CD43+CD93+ innate response activator (IRA) B cells was increased by E. coli LPS or P. gingivalis LPS treatment alone, whereas addition of CpG-ODN to E. coli LPS or P. gingivalis LPS significantly reduced the percentage of IRA B cells (Fig 3D). The percentage of CD43+CD93- B1 B cells was reduced only when treated with CpG-ODN together with E. coli LPS (Fig 3E).
Fig 3. Frequencies of Innate-like B cell subsets after E. coli LPS, P. gingivalis LPS and CpG-ODN treatment.
Splenocyte B cells were separated from WT mice and cultured 48 hours with E. coli LPS (10μg/ml), P. gingivalis LPS (10μg/ml), E. coli LPS (10μg/ml) + CpG (10μM), and P. gingivalis LPS (10μg/ml) + CpG (10μM). Cells were then stained by PE-conjugated anti IgM, PerCP-Cy5.5-conjugated anti CD23, APC-conjugated anti CD93, Pacific Blue-conjugated anti CD43 and measured by flow cytometry (A). Within overall innate-like B cell (IgMhighCD23low B cells) population, the percentage of CD43-CD93- marginal zone B cells (B), CD43-CD93+ transitional B cells (C), CD43+CD93+ innate response activator B cells (D) and CD43+CD93- B1 B cells (E) in different treatment groups were analyzed and compared respectively (mean±SE, n = 4, *p<0.05, **p<0.01).
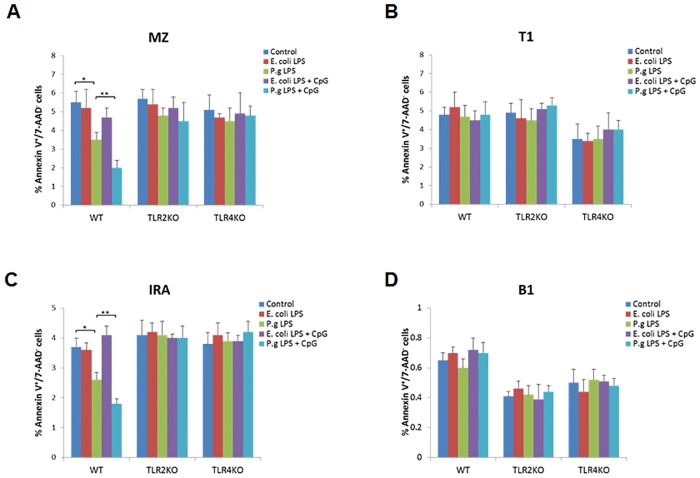
Suppression of Innate-like B cell apoptosis by P. gingivalis LPS and CpG-ODN
In order to determine the TLR-mediated regulation of innate-like B cell apoptosis, the percentage of AnnexinV+/7-AAD- (early apoptotic) B cells from each innate-like B cell subpopulation was evaluated after treatment with LPS and CpG-ODN. In WT mice, P. gingivalis LPS but not E. coli LPS significantly inhibited the percentage of AnnexinV+/7-AAD- B cells in MZ and IRA subpopulations (Fig 4A–4C), but not those in T1 or B1 subpopulations (Fig 4B–4D). In TLR2 KO or TLR4 KO mice, no differences were observed in the percentage of AnnexinV+/7-AAD- B cells in each innate-like B cell subpopulation (Fig 4A–4D) after different treatments.
Fig 4. Early apoptosis analysis of innate-like B cell subsets after E. coli LPS, P. gingivalis LPS and CpG-ODN treatment.
Splenocyte B cells were separated from WT, TLR2 KO and TLR4 KO mice and cultured 48 hours with E. coli LPS (10μg/ml), P. gingivalis LPS (10μg/ml), E. coli LPS (10μg/ml) + CpG (10μM), and P. gingivalis LPS (10μg/ml) + CpG (10μM). Cells were then stained by FITC-conjugated AnnexinV, 7-AAD, PE-conjugated anti IgM, PerCP-Cy5.5-conjugated anti CD23, APC-conjugated anti CD93, Pacific Blue-conjugated anti CD43 and measured by flow cytometry. In different innate-like B cell subsets including CD43-CD93- marginal zone B cells (A), CD43-CD93+ transitional B cells (B), CD43+CD93+ innate response activator B cells (C) and CD43+CD93- B1 B cells (D), the percentage of AnnexinV+/7-AAD- (early apoptotic) B cells in different treatment groups of WT, TLR2 KO and TLR4 KO mice were analyzed and compared respectively (mean±SE, n = 5, *p<0.05, **p<0.01).
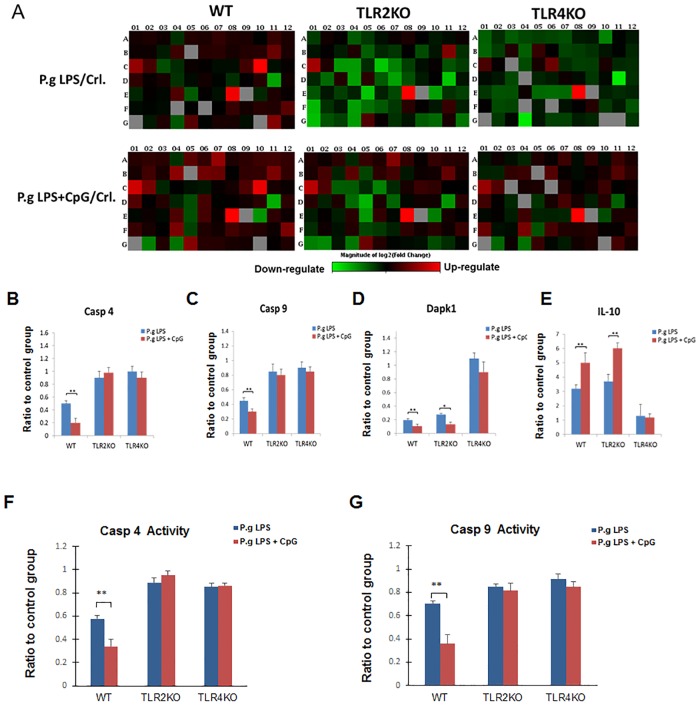
Regulation of apoptosis-related genes in B cells by P. gingivalis LPS and CpG-ODN
Gene Arrays were performed with RNA samples from spleen B cells isolated from WT, TLR2 KO and TLR4 KO mice and cultured 48 hours with P. gingivalis LPS (10μg/ml) and P. gingivalis LPS (10μg/ml) + CpG (10μM) (Fig 5A). Genes obtained from array results that were up-regulated or down-regulated by more than 2-fold relative to control were selected and individually verified with separate real-time PCR reactions using the same gene specific primers. The results showed that pro-apoptotic genes, Caspase 4 (Casp 4) and Caspase 9 (Casp 9) were down-regulated by P. gingivalis LPS in B cells from WT mice, but not B cells from TLR2 KO or TLR4 KO mice (Fig 5B and 5C). CpG-ODN further enhanced such down-regulation of Casp 4 and Casp 9 in B cells from WT mice (Fig 5B and 5C). Furthermore, pro-apoptotic genes, death-associated protein kinase 1 (Dapk1) was down-regulated by P. gingivalis LPS in B cells from WT and TLR2 KO mice, but not those from TLR4 KO mice (Fig 5D). However, anti-apoptotic gene interleukin 10 (IL-10) was up-regulated by P. gingivalis LPS in B cells from WT and TLR2 KO mice, but not from TLR4 KO mice (Fig 5E). The down-regulation of Dapk1 and up-regulation of IL-10 were further enhanced by addition of CpG-ODN in B cells from WT and TLR2 KO mice, but not B cells from TLR4 KO mice (Fig 5D and 5E). To further study the functional changes of pro-apoptotic genes, Casp4 and Casp9 proteins activities were investigated from total cell lysis of each group. Consistent with mRNA results (Fig 5B and 5C), Casp 4 and Casp 9 activities were down-regulated by P. gingivalis LPS in B cells from WT mice, but not from TLR2 KO or TLR4 KO mice (Fig 5F and 5G); CpG-ODN further enhanced such down-regulation of Casp 4 and Casp 9 activities in B cells from WT mice (Fig 5F and 5G).
Fig 5. Differential mRNA levels of apoptosis-related genes in B cells after P. gingivalis LPS and CpG-ODN treatment.
Splenocyte B cells were separated from WT, TLR2 KO and TLR4 KO mice and cultured 48 hours with P. gingivalis LPS (10μg/ml) and P. gingivalis LPS (10μg/ml) + CpG (10μM). Total RNA was extracted from these cells and used for RT² Profiler™ PCR Array Mouse Apoptosis (A). mRNA levels of Casp4 (B), Casp9 (C), Dapk1 (D) and IL-10 (E) in different groups of WT, TLR2 KO and TLR4 KO mice were determined by real-time PCR and the ratio of each treatment group to control group were analyzed and compared respectively (mean±SE, n = 3, *p<0.05, **p<0.01). Total cell lysis were used to detect Casp4 activity (F) and Casp9 activity (G) using fluorometric Assay kits. The ratio of each treatment group to control group were analyzed and compared respectively (mean±SE, n = 3, *p<0.05, **p<0.01).
Discussion
B lymphocytes are the predominant cells in established and advanced periodontal lesions, contributing to the B cell-mediated immune defenses as well as periodontal pathogenesis. However, the role of TLR signaling on B cells during periodontal diseases is not fully understood. In the present study, we have determined whether innate-like B cell apoptosis could be regulated by TLR ligands from periodontal pathogens.
In this study we elected to use high concentration of LPS in our cell culture experiments to test the effect of P. gingivalis LPS and E. coli LPS on B cell proliferation and apoptosis. We have previously tested extensively the dose response of cultured purified B cells to both P. gingivalis LPS and E. coli LPS in WT, TLR2 KO and TLR4 KO mice. Our results indicated that higher concentration of LPS (10ug/mL), especially P. gingivalis LPS, is needed to effectively stimulate purified B cell responses in the absence of other cells (S1 Fig). The purified B cells do not respond well to LPS probably due to the lacking of T cell help [30] and B cells from TLR2 and TLR4 KO mice respond poorly to LPS due to their deficiency in TLR2/4 signaling. Moreover, P. gingivalis LPS showed less induction on proliferation and stronger inhibition on apoptosis than E. coli LPS, suggesting a complexity of links between cell proliferation and apoptosis in B cells. It has been showed that cell proliferation and apoptosis may address both positive relationship [31, 32] and negative relationship [33] due to cell type, cellular environment and genetic background [34, 35] and further study is needed to investigate the links between proliferation and apoptosis in B cells.
We demonstrated that P. gingivalis LPS- or E. coli LPS-induced B cell proliferation was enhanced by CpG-ODN. However, B cell proliferation was differentially regulated by P. gingivalis LPS as compared to E. coli LPS. LPS derived from the periodontal pathogen P. gingivalis has been shown to differ from E. coli LPS in structure and function; therefore, triggering different intracellular inflammatory signaling pathways [36]. Studies have suggested that P. gingivalis LPS and E. coli LPS differently regulate cytokine production in human gingival fibroblasts [37]. E. coli LPS, but not P. gingivalis LPS stimulates IL-6 production of periodontal ligament cell [38]. Furthermore, the tetra- and penta-acylated lipid A structures of P. gingivalis LPS differentially activate TLR4-mediated NF-kappa B signaling pathway, and significantly modulate the expression of IL-6 and IL-8 in human gingival fibroblasts [39]. Our results indicated that P. gingivalis LPS, but not E. coli LPS suppressed the early and late apoptosis of B cells, which could be enhanced by CpG-ODN (Fig 2). It has long been recognized that stimulatory CpG-ODN has anti-apoptotic effect on B cells [40–42], indicating that CpG can act independently against cell apoptosis. However, our results showed that CpG-ODN and LPS induced anti-apoptotic effects involve common TLR signaling pathways (TLR2/4). Addition of CpG further enhanced gene expression profiles observed in LPS-treated group in WT but not TLR2/4 KO mice (Fig 5). This suggests that CpG-ODN induced enhancement of anti-apoptotic effect could be achieved through both LPS-dependent and independent mechanisms, which will be important to be addressed in future studies.
Recent studies have shown that P. gingivalis could manipulate TLR signaling and subvert leukocytes to create a favorable environment for a select community of bacteria that, in turn, adversely affects the periodontal tissues [43, 44]. Thus, this TLR ligands- induced dysregulation of apoptosis in B cells may cause autoimmune manifestations.
Our findings indicated that MZ B cells and IRA B cells were the predominant innate-like B cell subsets that their spontaneous programmed death was suppressed by P. gingivalis LPS and CpG-ODN. MZ B cell subset is critical for antibody-mediated protection against bacterial and viral infections at relatively early stages of infection [45]. Compared with follicular (FO) B cells, MZ B cells are more readily activated upon TLR stimulation [46]. These properties enable MZ B cells with an important role in host defense at the early stages of an innate immune response as well as adaptive immune response [19, 47]. IRA B cells are a recently identified effector B cell population that is functionally distinctive from B1a B cells and protects against microbial sepsis [18]. While sustained innate response can be protective [48] as well as pathogenic [49], further in vivo investigations are needed to determine whether disruption of B cell apoptosis could be another mechanism for Porphyromonas gingivalis to uncouple bacterial clearance from inflammation.
Casp 4 and Casp 9 are protease enzymes playing essential roles in programmed cell death (including apoptosis, pyroptosis and necroptosis) and inflammation [50, 51]. P. gingivalis LPS and P. gingivalis LPS + CpG-ODN significantly decreased the mRNA of Casp 4 and Casp 9 in B cells of WT mice but not of TLR2 KO and TLR4 KO mice, suggesting the inhibition of B cell apoptosis by P. gingivalis LPS and CpG-ODN was depended on both TLR2 and TLR4. However, Dapk1, a positive mediator of gamma-interferon induced programmed cell death [52], showed similar reduction in TLR2 KO mice not TLR4 KO mice compared with WT mice after P. gingivalis LPS and P. gingivalis LPS + CpG-ODN treatment. These results suggest that TLR4, but not TLR2, is essential to regulate Dapk1 in B cells by stimulation with P. gingivalis LPS and CpG-ODN. Moreover, up-regulation of IL-10 was also in TLR4-dependent manner. Thus, TLR2 and TLR4 signaling were differentially involved in regulating Casp 4/Casp 9 and Dapk1/IL-10 and their underlying mechanisms need to be further investigated.
In summary, our results provided new information about multiple TLR signaling on the control of innate-like B cell-apoptosis and may contribute to the development of therapeutic strategies that are effective in preventing and/or reducing periodontal disease pathogenesis.
Supporting Information
Purified B cells (2×105/well) were cultured in 200μl complete medium in 96-well plate for 2 days in the presence of P. gingivalis LPS or E. coli LPS (200ng, 2μg and 10μg/ml). MTS reagent was added (40μl/well) 4 hours before the termination of the experiment using a CellTiter 96 AQueous Assay kit (Promega Corp). After 4 hour incubation, the plate was read at OD 490nm using a microplate reader (BioTek). The absorbance of the formazan at 490nm was measured as an indication of cell proliferation. N = 3.
(PDF)
Acknowledgments
We thank Zhihan Li for her technical assistance in flow cytometry analysis. We are also grateful for Dr. Jun-O Jin from the Department of Immunology and Infectious Diseases, Forsyth Institute for his sharing of his research experiences in carrying out some experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
National Natural Science Foundation of China 81470740 to X Yu; National Institute of Dental and Craniofacial Research R21DE021837 and R56DE023807 to X Han.
References
- 1.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annual review of immunology. 2006;24:353–89. Epub 2006/03/23. 10.1146/annurev.immunol.24.021605.090552 . [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5(10):987–95. Epub 2004/09/30. 10.1038/ni1112 . [DOI] [PubMed] [Google Scholar]
- 3.Bohnhorst J, Rasmussen T, Moen SH, Flottum M, Knudsen L, Borset M, et al. Toll-like receptors mediate proliferation and survival of multiple myeloma cells. Leukemia. 2006;20(6):1138–44. Epub 2006/04/18. 10.1038/sj.leu.2404225 . [DOI] [PubMed] [Google Scholar]
- 4.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178(12):7779–86. Epub 2007/06/06. . [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101(11):4500–4. Epub 2003/02/01. 10.1182/blood-2002-11-3569 . [DOI] [PubMed] [Google Scholar]
- 6.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Pozzetto B, Richard Y, et al. Identification of two subpopulations of purified human blood B cells, CD27- CD23+ and CD27high CD80+, that strongly express cell surface Toll-like receptor 9 and secrete high levels of interleukin-6. Immunology. 2008;125(3):430–7. Epub 2008/05/01. 10.1111/j.1365-2567.2008.02844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorencini M, Silva JA, Almeida CA, Bruni-Cardoso A, Carvalho HF, Stach-Machado DR. A new paradigm in the periodontal disease progression: gingival connective tissue remodeling with simultaneous collagen degradation and fibers thickening. Tissue & cell. 2009;41(1):43–50. Epub 2008/09/20. 10.1016/j.tice.2008.07.001 . [DOI] [PubMed] [Google Scholar]
- 8.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes and infection / Institut Pasteur. 2000;2(10):1181–92. Epub 2000/09/29. . [DOI] [PubMed] [Google Scholar]
- 9.Maddi A, Scannapieco FA. Oral biofilms, oral and periodontal infections, and systemic disease. American journal of dentistry. 2013;26(5):249–54. Epub 2014/02/01. . [PubMed] [Google Scholar]
- 10.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Molecular oral microbiology. 2012;27(6):409–19. Epub 2012/11/09. 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bainbridge BW, Darveau RP. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta odontologica Scandinavica. 2001;59(3):131–8. Epub 2001/08/15. . [DOI] [PubMed] [Google Scholar]
- 12.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infection and immunity. 2004;72(9):5041–51. Epub 2004/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allman D, Pillai S. Peripheral B cell subsets. Current opinion in immunology. 2008;20(2):149–57. Epub 2008/04/25. 10.1016/j.coi.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, et al. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer immunology, immunotherapy: CII. 2013;62(1):87–99. Epub 2012/07/10. 10.1007/s00262-012-1313-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Current opinion in immunology. 2001;13(2):195–201. Epub 2001/03/03. . [DOI] [PubMed] [Google Scholar]
- 16.Xie H, Hong J, Sharma A, Wang BY. Streptococcus cristatus ArcA interferes with Porphyromonas gingivalis pathogenicity in mice. Journal of periodontal research. 2012;47(5):578–83. Epub 2012/03/28. 10.1111/j.1600-0765.2012.01469.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zouali M, Richard Y. Marginal zone B-cells, a gatekeeper of innate immunity. Frontiers in immunology. 2011;2:63 Epub 2011/01/01. 10.3389/fimmu.2011.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335(6068):597–601. Epub 2012/01/17. 10.1126/science.1215173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nature reviews Immunology. 2013;13(2):118–32. Epub 2013/01/26. 10.1038/nri3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearney JF. Innate-like B cells. Springer seminars in immunopathology. 2005;26(4):377–83. Epub 2005/01/13. 10.1007/s00281-004-0184-0 . [DOI] [PubMed] [Google Scholar]
- 21.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell proliferation. 2012;45(6):487–98. Epub 2012/10/04. 10.1111/j.1365-2184.2012.00845.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fanzo JC, Hu CM, Jang SY, Pernis AB. Regulation of lymphocyte apoptosis by interferon regulatory factor 4 (IRF-4). The Journal of experimental medicine. 2003;197(3):303–14. Epub 2003/02/05. 10.1084/jem.20020717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Flaherty E, Wong WK, Pettit SJ, Seymour K, Ali S, Kirby JA. Regulation of T-cell apoptosis: a mixed lymphocyte reaction model. Immunology. 2000;100(3):289–99. Epub 2000/08/06. 10.1046/j.1365-2567.2000.00048.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Hoyos M, Carrio R, Merino J, Merino R. Regulation of B cell apoptosis by Bcl-2 and Bcl-XL and its role in the development of autoimmune diseases (Review). International journal of molecular medicine. 1998;1(2):475–83. Epub 1998/12/16. . [DOI] [PubMed] [Google Scholar]
- 25.Bi W, Zhu L, Jing X, Zeng Z, Liang Y, Xu A, et al. Rifampicin improves neuronal apoptosis in LPS-stimulated cocultured BV2 cells through inhibition of the TLR-4 pathway. Molecular medicine reports. 2014;10(4):1793–9. Epub 2014/08/15. 10.3892/mmr.2014.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C, et al. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod. 2011;26(10):2799–806. Epub 2011/07/22. 10.1093/humrep/der234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boffey J, Nicholl D, Wagner ER, Townson K, Goodyear C, Furukawa K, et al. Innate murine B cells produce anti-disialosyl antibodies reactive with Campylobacter jejuni LPS and gangliosides that are polyreactive and encoded by a restricted set of unmutated V genes. Journal of neuroimmunology. 2004;152(1–2):98–111. Epub 2004/06/30. 10.1016/j.jneuroim.2004.04.002 . [DOI] [PubMed] [Google Scholar]
- 28.Moore-Connors JM, Kim HS, Marshall JS, Stadnyk AW, Halperin SA, Wang J. CD43-, but not CD43+, IL-10-producing CD1dhiCD5+ B cells suppress type 1 immune responses during Chlamydia muridarum genital tract infection. Mucosal immunology. 2015;8(1):94–106. Epub 2014/06/19. 10.1038/mi.2014.45 . [DOI] [PubMed] [Google Scholar]
- 29.de Andres B, Prado C, Palacios B, Alia M, Jagtap S, Serrano N, et al. Dynamics of the splenic innate-like CD19(+)CD45Rlo cell population from adult mice in homeostatic and activated conditions. J Immunol. 2012;189(5):2300–8. Epub 2012/07/28. 10.4049/jimmunol.1200224 . [DOI] [PubMed] [Google Scholar]
- 30.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. Journal of immunology. 2015;194(4):1395–401. 10.4049/jimmunol.1401329 . [DOI] [PubMed] [Google Scholar]
- 31.Reid S, Ritchie A, Boring L, Gosling J, Cooper S, Hangoc G, et al. Enhanced myeloid progenitor cell cycling and apoptosis in mice lacking the chemokine receptor, CCR2. Blood. 1999;93(5):1524–33. . [PubMed] [Google Scholar]
- 32.Traver D, Akashi K, Weissman IL, Lagasse E. Mice defective in two apoptosis pathways in the myeloid lineage develop acute myeloblastic leukemia. Immunity. 1998;9(1):47–57. . [DOI] [PubMed] [Google Scholar]
- 33.Koury MJ. Programmed cell death (apoptosis) in hematopoiesis. Experimental hematology. 1992;20(4):391–4. . [PubMed] [Google Scholar]
- 34.Alenzi FQ. Links between apoptosis, proliferation and the cell cycle. British journal of biomedical science. 2004;61(2):99–102. . [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell proliferation. 2003;36(3):165–75. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diya Z, Lili C, Shenglai L, Zhiyuan G, Jie Y. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate immunity. 2008;14(2):99–107. Epub 2008/08/21. 10.1177/1753425907088244 . [DOI] [PubMed] [Google Scholar]
- 37.Andrukhov O, Ertlschweiger S, Moritz A, Bantleon HP, Rausch-Fan X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta odontologica Scandinavica. 2014;72(5):337–45. Epub 2013/11/22. 10.3109/00016357.2013.834535 . [DOI] [PubMed] [Google Scholar]
- 38.Nebel D, Arvidsson J, Lillqvist J, Holm A, Nilsson BO. Differential effects of LPS from Escherichia coli and Porphyromonas gingivalis on IL-6 production in human periodontal ligament cells. Acta odontologica Scandinavica. 2013;71(3–4):892–8. Epub 2012/11/03. 10.3109/00016357.2012.734415 . [DOI] [PubMed] [Google Scholar]
- 39.Herath TD, Darveau RP, Seneviratne CJ, Wang CY, Wang Y, Jin L. Tetra- and penta-acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated NF-kappaB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PloS one. 2013;8(3):e58496 Epub 2013/04/05. 10.1371/journal.pone.0058496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Karras JG, Colarusso TP, Foote LC, Rothstein TL. Unmethylated CpG motifs protect murine B lymphocytes against Fas-mediated apoptosis. Cellular immunology. 1997;180(2):162–7. Epub 1997/10/28. 10.1006/cimm.1997.1156 . [DOI] [PubMed] [Google Scholar]
- 41.Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998;160(12):5898–906. Epub 1998/06/24. . [PubMed] [Google Scholar]
- 42.Yi AK, Peckham DW, Ashman RF, Krieg AM. CpG DNA rescues B cells from apoptosis by activating NFkappaB and preventing mitochondrial membrane potential disruption via a chloroquine-sensitive pathway. International immunology. 1999;11(12):2015–24. Epub 1999/12/11. . [DOI] [PubMed] [Google Scholar]
- 43.Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell host & microbe. 2014;15(6):768–78. Epub 2014/06/13. 10.1016/j.chom.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenobia C, Hajishengallis G. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence. 2015;6(3):236–43. Epub 2015/02/06. 10.1080/21505594.2014.999567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180(6):3882–8. Epub 2008/03/07. . [DOI] [PubMed] [Google Scholar]
- 46.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. European journal of immunology. 1997;27(9):2366–74. Epub 1997/10/28. 10.1002/eji.1830270935 . [DOI] [PubMed] [Google Scholar]
- 47.Rosado MM, Scarsella M, Cascioli S, Giorda E, Carsetti R. Purification and immunophenotypic characterization of murine MZ and T2-MZP cells. Methods Mol Biol. 2014;1190:3–16. Epub 2014/07/13. 10.1007/978-1-4939-1161-5_1 . [DOI] [PubMed] [Google Scholar]
- 48.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179(6):3926–36. Epub 2007/09/06. . [DOI] [PubMed] [Google Scholar]
- 49.Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, Garcia-Sastre A, et al. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3455–60. Epub 2009/02/17. 10.1073/pnas.0813234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinch LN, Shi S, Cheng H, Cong Q, Pei J, Mariani V, et al. CASP9 target classification. Proteins. 2011;79 Suppl 10:21–36. 10.1002/prot.23190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. European journal of immunology. 2015;45(10):2911–7. 10.1002/eji.201545523 . [DOI] [PubMed] [Google Scholar]
- 52.Yoo HJ, Byun HJ, Kim BR, Lee KH, Park SY, Rho SB. DAPk1 inhibits NF-kappaB activation through TNF-alpha and INF-gamma-induced apoptosis. Cellular signalling. 2012;24(7):1471–7. 10.1016/j.cellsig.2012.03.010 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purified B cells (2×105/well) were cultured in 200μl complete medium in 96-well plate for 2 days in the presence of P. gingivalis LPS or E. coli LPS (200ng, 2μg and 10μg/ml). MTS reagent was added (40μl/well) 4 hours before the termination of the experiment using a CellTiter 96 AQueous Assay kit (Promega Corp). After 4 hour incubation, the plate was read at OD 490nm using a microplate reader (BioTek). The absorbance of the formazan at 490nm was measured as an indication of cell proliferation. N = 3.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.