Abstract
ARS-Media for Excel is an ion solution calculator that uses “Microsoft Excel” to generate recipes of salts for complex ion mixtures specified by the user. Generating salt combinations (recipes) that result in pre-specified target ion values is a linear programming problem. Excel’s Solver add-on solves the linear programming equation to generate a recipe. Calculating a mixture of salts to generate exact solutions of complex ionic mixtures is required for at least 2 types of problems– 1) formulating relevant ecological/biological ionic solutions such as those from a specific lake, soil, cell, tissue, or organ and, 2) designing ion confounding-free experiments to determine ion-specific effects where ions are treated as statistical factors. Using ARS-Media for Excel to solve these two problems is illustrated by 1) exactly reconstructing a soil solution representative of a loamy agricultural soil and, 2) constructing an ion-based experiment to determine the effects of substituting Na+ for K+ on the growth of a Valencia sweet orange nonembryogenic cell line.
Introduction
Calculating a mixture of salts (‘recipe’) to generate exact solutions of complex ionic solution mixtures is required for at least 2 types of problems–
1. Formulating Relevant Ecological/Biological Ionic Solutions
Reconstructing the ionic composition of ecological and biological fluid samples such as those from a specific lake, soil, cell, tissue, or organ requires a method to determine the type and concentration of salts to use to formulate the recipes. Some examples of studies that attempted to reconstruct these types of compositions include fresh water lakes [1], in vitro growth and production of the glycoalkaloid solamargin [2], micropropagation of yellow passion fruit on a medium based on analysis of healthy young leaves [3], soil solution-based microbial culture medium [4], conifer suspension medium based on seed composition [5], micropropagation of hazelnut on a medium based on seed composition [6], in vitro rooting of the carob tree on a medium based on leaf composition [7], micropropagation of Galanthus species on a medium based on bulb composition [8], micropropagation of Persian walnut on a medium based on in vitro explant composition [9], micropropagation of almond, Prunus dulcis, based on the mineral composition of raw almond kernels [10], shoot organogenesis of Eucalyptus dunnii based on the mineral composition of young stump shoots [11]. No examples were found where the resulting recipe reconstructed the composition precisely. A well-documented example of the difficulty of formulating complex ionic solutions is the study of Angle et al. [4], who reported the development of a growth medium based on the ionic composition of a soil solution representative of “loamy agricultural soils with a neutral pH”. They explain that most culture media formulations for culturing soil organisms “do not resemble the environment from which the microorganisms were isolated”, and this creates the possibility that “both genetic and phenotypic properties of microorganisms growing on these media may be different from those of the same microorganisms growing in soil.” Their extensive manipulations to recreate the soil solution are well-described, but the soil solution was only approximated, not recreated. The authors stated, “Although this medium is a much better approximation of the composition of the soil solution than previous media, concentrations of several ions deviate from those in the soil solution more than we had hoped.”
2. Design of Ion Confounding-Free Experiments to Determine Ion-Specific Effects
Determining the effects of specific ions such as NO3-, NH4+, PO43-, K+, SO42-, Ca2+, Mg2+, and Cl- requires experiments designed where ions rather than salts are treated as statistical factors or mixture components. Because ion-based experiments are free of ion-confounding, ion-specific effects can be determined [12, 13]. Varying ions that are not part of the experiment results in ion-confounding. This occurs when the objective of the experiment is to determine the effects of a specific ion(s) but salts are varied. For example, determining the effect of K+ by varying KCl confounds the effect of K+ with the effect of Cl- and the effect of the interaction of K+ x Cl-. Because the treatments in ion-based experiments are various combinations of ions, each treatment requires a recipe of the salts necessary to achieve these combinations. Recipes are required to actually setup the experiment. Constructing these recipes “by hand” is quite difficult and probably why nearly all reports on the effects of specific ions use salts, and consequently exhibit ion confounding.
For example, a recent paper by Krishnasamy et al. [14] tried to determine the effects of K+ and Na+ on four wheat cultivars that varied in their differential productivity on sodic and K-deficient soils. The objective was to improve K fertilization on these soils based on the K-use efficiency of the cultivar. The study varied the 2 salts KCl and NaCl to determine the specific effects of K+ and Na+. This approach confounded the effects of K+ and Na+ with those of Cl- (Table 1). The specific effects of K+ with Na+, or their interaction with Cl-, cannot be determined. Further, using only Cl- to determine the effects of K+ with Na+ assumes that the anion used, other than contributing a charge, has no effect. Are the effects of K+ with Na+ the same regardless of the anion used? Would the effects of K+ with Na+ be the same if NO3-, SO42-, or acetate- were varied rather than Cl-? If the specific effects of K+ with Na+ cannot be determined, what can be determined from this type of study? The two salts are the independent factors so the main effects of KCl and NaCl, and their interaction, KCl x NaCl, can be determined. The study compares salts, not ions. The conclusions of the study about the specific effects of K+ and Na+ may be true, but such conclusions cannot be derived from the experiment. Unfortunately, unlike this study, many studies provide insufficient or unclear information needed to deconstruct the treatments into their ion compositions [15, 16]. Designing an experiment to determine the effects of K+ and Na+ requires varying only K+ and Na+ and holding constant all other ion levels. Including various anions as factors or components would extend the experiment.
Table 1. Deconstructing the experiment of Krishnasamy et al. [14] to determine the effects of K+ and Na+ into the actual ion levels.
The experiment varied three ions K+, Na+, and Cl-. The effects of K+ and Na+ are confounded with those of Cl-.
| Salts varied | Final Ion Composition | Total mM | |||||
|---|---|---|---|---|---|---|---|
| Treatments | KCl | NaCl | K+ | Na+ | Cl- | ∑ mM | pH* |
| mM | mM | mM | mM | mM | mM | ||
| 1 | 0.54 | 0 | 0.54 | 0 | 0.54 | 1.08 | 5.6 |
| 2 | 0.54 | 1.1 | 0.54 | 1.1 | 1.64 | 3.28 | 5.6 |
| 3 | 0.54 | 2.2 | 0.54 | 2.2 | 2.74 | 5.48 | 5.6 |
| 4 | 0.54 | 4.3 | 0.54 | 4.3 | 4.84 | 9.68 | 5.6 |
| 5 | 0.54 | 8.7 | 0.54 | 8.7 | 9.24 | 18.48 | 5.6 |
| 6 | 1.34 | 0 | 1.34 | 0 | 1.34 | 2.68 | 5.6 |
| 7 | 1.34 | 1.1 | 1.34 | 1.1 | 2.44 | 4.88 | 5.6 |
| 8 | 1.34 | 2.2 | 1.34 | 2.2 | 3.54 | 7.08 | 5.6 |
| 9 | 1.34 | 4.3 | 1.34 | 4.3 | 5.64 | 11.28 | 5.6 |
| 10 | 1.34 | 8.7 | 1.34 | 8.7 | 10.04 | 20.08 | 6.9 |
* Calculated using the chemical equilibrium software MINEQL+ [17].
This paper describes the utility, validation, and availability of an ion solution calculator, ARS-Media for Excel that uses “Microsoft Excel” to generate recipes of salts that, when dissolved, result in the complex ion mixtures specified by the user.
Materials and Methods
ARS-Media for Excel uses “Microsoft Excel (2013)” (herein referred to as Excel) to solve Eq (1) to generate recipes of salts for complex ion mixtures specified by the user.
| (1) |
where c is a known weighting coefficient, and X is the unknown amount of an individual salt,
where a are known coefficients of ion proportionality for each salt X that contributes an ion defined by the target ion concentration b; a non-negative constraint ensures positive values. Eq (1) results in a list of the type and amount of salts to achieve the exact ion concentrations specified by b.
ARS-Media for Excel provides the universality and convenience of a spreadsheet but with additional functionality compared to an earlier Window-based, ARS-Media, desktop application [12]. The added functionality is a result of “Microsoft Excel’s” Solver Add-in program (Frontline Systems, USA) and its more extensive set of options. For example, forcing Eq (1) to include specific salts at specified concentrations in the final recipe is now possible. The calculations of using Eq (1), with examples, is described in detail by Niedz and Evens (2006) [12]. The spreadsheet is formula-based and uses no macros because they sometimes conflict with institutional IT security systems. ARS-Media for Excel is free and available by download, along with instructions, from the U.S. Department of Agriculture, Agricultural Research Service software site as ARS-Media for Excel.
Utility and Validation
The utility of ARS-Media for Excel is that it solves 2 problems common in experimental biology, 1) formulating relevant ecological/biological ionic solutions, and 2) design of ion confounding-free experiments to determine ion-specific effects. An example of using ARS-Media for Excel to solve each of these problems is provided.
Formulating relevant ecological/biological ionic solutions
An exact formulation of the soil solution that Angle et al. [4] attempted to construct, as described above, was calculated using ARS-Media for Excel. The ion values entered into ARS-Media for Excel are those listed in Table 1 of the Angle et al. [4] paper (Table 2).
Table 2. Ionic compositions of the “soil solution equivalent” (SSE) and ARS-Media for Excel formulation and how each compares to the ion levels of the target soil solution.
| Ion | Soil solution | SSE | % Deviation of SSE from soil solution | ARS-Media for Excel formulation | % Deviation of ARS-Media for Excel from soil solution |
|---|---|---|---|---|---|
| mM | mM | mM | |||
| NO3 | 4.7 | 2.5 | - 47 | 4.7 | 0 |
| NH4 | 0.4 | 2.5 | + 525 | 0.4 | 0 |
| HPO4 | 0.005 | 0.005 | 0 | 0.005 | 0 |
| Na | 1.4 | 2.5 | + 79 | 1.4 | 0 |
| Ca | 12.4 | 4.0 | - 68 | 12.4 | 0 |
| Mg | 3.4 | 2.0 | - 41 | 3.4 | 0 |
| K | 0.3 | 0.503 | + 68 | 0.3 | 0 |
| Cl | 1.9 | 4.0 | + 111 | 1.9 | 0 |
| SO4 | 3.4 | 5.0 | + 47 | 3.4 | 0 |
| Fe | 0.02 | 0.2 | + 900 | 0.02 | 0 |
Design of ion confounding-free experiments to determine ion-specific effects
To illustrate the use of ARS-Media for Excel for ion experimentation, an experiment free of ion confounding to determine the specific effects of K+ and Na+ on the growth of a Valencia sweet orange nonembryogenic cell line was conducted.
Tissue Source
A nonembryogenic cell line of Valencia sweet orange was developed and maintained as described [18] (Fig 1). Briefly, the line was subcultured every 30 days onto MT medium [19] supplemented with 1-μM 2,4-dichlorophenoxyacetic acid (2,4-D), 1-μM 6-benzylaminopurine (BA), and 100 mg l−1 casein hydrolysate. MT medium includes the inorganic salts of MS medium [20]. The cultures were grown in a growth cabinet under low light (15–20 μmol m−2 s−1), provided by cool-white fluorescent lamps, constant 27°C, and a 4-h photoperiod.
Fig 1. Nonembryogenic citrus cell line of Valencia sweet orange.
Experimental Design
The experiment was a 2-component quadratic mixture design with D-optimal point selection (Table 2). Mixture designs vary the proportion of the components but fix the amount. Thus, the approach was to determine the effect of substituting K+ with Na+ on fresh weight growth of a nonembryogenic cell line of Valencia sweet orange. The cell line was grown on MS salts where the proportion of [K+] and [Na+] were varied while 1) maintaining the MS [K+ + Na+] of 23.2 mM, and 2) holding all other ions of MS medium constant. The resulting experiment varied only K+ + Na+ and therefore free of ion confounding (Table 3). A total of 23.2 mM was selected because the total [K+ + Na+] of MS medium is 20.25 mM. However, adjusting pH under our conditions to 5.8 using NaOH requires an additional 2.95 mM Na+. Thus, adding 2.95 mM Na+ results in formulations with the target pH of 5.8 without the pH adjustment step. The final design included 13 points (3 model, 2 lack-of-fit, and 8 replicates to estimate pure error) and was sufficient to model a quadratic polynomial.
Table 3. The five unique formulations that comprised the experiment.
The experiment was free of ion confounding because only the target ions Na+ and K+ were varied. All other ions in MS medium were held constant.
| Media | Na+ | K+ | NH4+ | NO3- | PO43- | Ca2+ | Mg2+ | SO42- | Cl- | ∑ mM |
|---|---|---|---|---|---|---|---|---|---|---|
| mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | |
| 1 | 23.2 | 0 | 20 | 40 | 1.25 | 3 | 1.5 | 1.6 | 6 | 96.55 |
| 2 | 17.4 | 5.8 | 20 | 40 | 1.25 | 3 | 1.5 | 1.6 | 6 | 96.55 |
| 3 | 11.6 | 11.6 | 20 | 40 | 1.25 | 3 | 1.5 | 1.6 | 6 | 96.55 |
| 4 | 5.8 | 17.4 | 20 | 40 | 1.25 | 3 | 1.5 | 1.6 | 6 | 96.55 |
| 5 | 0 | 23.2 | 20 | 40 | 1.25 | 3 | 1.5 | 1.6 | 6 | 96.55 |
One response was measured, fresh weight growth. The initial amount of tissue cultured onto each dish was approximately 0.68 g (± 0.07 S.D.). The tissue was cultured for 14 days, the final weight measured, and the fresh weight growth calculated as the percent increase over the initial culture weight. Six culture dishes were used to estimate the response, as an average, at each treatment design point (Table 1). Thus, the experiment utilized a total of 78 culture dishes (13 treatment design points x 6 dishes per design point). A polynomial model and ANOVA were generated. Model adequacy tests were conducted as described by Anderson and Whitcomb (2005) [21] and included an analysis of the normal probability plot of studentized residuals to determine if data transformation was required. Data transformation was not required. The statistical software used was Design Expert® 9 (Stat-Ease, Inc, Minneapolis, MN).
ARS-Media for Excel calculated the recipes for the 5 unique formulations (3 model and 2 lack-of-fit points) that comprised the experiment. The mM ion levels for each of the 5 formulations used in the design (Table 3) were entered into ARS-Media for Excel and recipes calculated using Excel’s Solver function (Table 4). To simplify the illustration, not all of the inorganic mineral nutrient ions in MS medium were included in the calculations. The ions Cl-, Fe3+, Mn2+, Zn2+, BO33-, I-, Cu2+, MoO42-, and Co2+ were added to each formulation using the salts and amounts used for MS medium.
Table 4. Recipes generated by ARS-Media for Excel for the 5 media listed in Table 2.
In addition to the salts listed, all recipes included the MS salts that deliver Fe3+, Mn2+, Zn2+, BO33-, I-, Cu2+, MoO42-, and Co2+ as follows: FeSO4.7H2O, Na2EDTA.2H2O, MnSO4.4H2O, ZnSO4.4H2O, H3BO3, KI, CuSO4.5H2O, Na2MoO4.2H2O, and CoCl2.6H2O. These salts did not vary between formulations.
| Media | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Salts | K+ (mM) | 23 | 17.3 | 11.6 | 5.9 | 0.2 |
| Na+ (mM) | 0.2 | 5.9 | 11.6 | 17.3 | 23 | |
| mg/L | mg/L | mg/L | mg/L | mg/L | ||
| (NH4)2SO4 | 13 | - | - | - | - | |
| CaCl2.2H2O | 441 | 441 | 441 | 441 | 441 | |
| KH2PO4 | 170 | 51 | 51 | 51 | 27 | |
| KNO3 | 2199 | 1711 | 1135 | 559 | - | |
| MgSO4.7H2O | 370 | 370 | 370 | 370 | 370 | |
| Na2SO4 | - | 14 | 14 | 14 | 14 | |
| Na3PO4 | - | 143 | 143 | 143 | 143 | |
| NaH2PO4 | - | - | - | - | 21 | |
| NaNO3 | - | 261 | 746 | 1230 | 1700 | |
| NaOH | 8 | - | - | - | - | |
| NH4NO3 | 1461 | 1601 | 1601 | 1601 | 1601 | |
| NH4OH | 54 | - | - | - | - | |
Results
Formulating Relevant Ecological/Biological Ionic Solutions
Using ARS-Media for Excel to solve for the combination of salts to construct a recipe for the specified soil solution (Table 5) resulted in a formulation (Table 6) that exactly achieves each of the soil solution ion levels (Table 5). The ARS-Media for Excel solution is exact.
Table 5. Treatment design points and fresh weight growth data of a 2-component quadratic mixture design.
The proportions of K+ and Na+ were varied while the total amount was kept constant at 23.2 mM, the level in MS medium. For example, treatment point #1 would include 17.4 mM Na+ and 5.8 mM K+.
| Treatment Design Points | Na+ | K+ | Fresh weight growth |
|---|---|---|---|
| proportions | % | ||
| 1 | 0.75 | 0.25 | 1038 |
| 2 | 0.5 | 0.5 | 1082 |
| 3 | 1 | 0 | 217 |
| 4 | 1 | 0 | 196 |
| 5 | 0.5 | 0.5 | 1317 |
| 6 | 0.25 | 0.75 | 1031 |
| 7 | 0.75 | 0.25 | 906 |
| 8 | 0 | 1 | 867 |
| 9 | 1 | 0 | 215 |
| 10 | 0 | 1 | 1125 |
| 11 | 0.5 | 0.5 | 1021 |
| 12 | 0 | 1 | 1146 |
| 13 | 0.25 | 0.75 | 1209 |
Table 6. Recipe generated by ARS-Media for Excel to recreate the soil solution ion composition of Angle et al. [4].
| Salts | Amount |
|---|---|
| mg L-1 | |
| (NH4)2SO4 | 26.43005 |
| Ca(OH)2 | 16.67094 |
| CaSO4*2H2O | 391.7074 |
| K2SO4 | 26.139 |
| Mg(NO3)2*6H2O | 602.6105 |
| MgCl2*6H2O | 193.164 |
| MgSO4*7H2O | 24.64998 |
| Na2FeEDTA*2H2O | 8.521155 |
| Na2HPO4 | 0.7098 |
| Na2SO4 | 95.8902 |
Design of Ion Confounding-Free Experiments to Determine Ion-Specific Effects
Tissue was harvested, weighed, and the difference between the final and initial fresh weights calculated to determine the percent increase in fresh weight. The ANOVA revealed significant linear and quadratic effects (Table 7).
Table 7. ANOVA of the effect of K+ and Na+ on fresh weight growth of citrus nonembryogenic cells.
The coefficients for K+ and Na+ under the linear mixture are estimates of the response at each vertex, not estimates of the effects of these two ions. The K+ x Na+ term is not an interaction term, though it looks like one, but a quadratic blending term unique to mixture models. This term is used to determine if the mixture components exhibit nonlinear blending and if that blending is synergistic or antagonistic.
| Source | Sum of Squares | df | Mean Squares | F Value | p-value Prob > F | Coefficient Estimate |
|---|---|---|---|---|---|---|
| Model | 1.70E+06 | 2 | 8.51E+05 | 45 | <0.0001 | |
| Linear mixture | 1.01E+06 | 1 | 1.01E+06 | 54 | <0.0001 | |
| K+ | 1011 | |||||
| Na+ | 251 | |||||
| K+ x Na+ | 6.92E+05 | 1 | 6.92E+05 | 37 | 0.0001 | 2112 |
| Residual | 1.87E+05 | 10 | 1.87E+04 | |||
| Lack of Fit | 6.53E+04 | 2 | 3.26E+04 | 2.14 | 0.1804 | |
| Pure Error | 1.22E+05 | 8 | 1.53E+04 | |||
| Cor Total | 1.89E+06 | 12 |
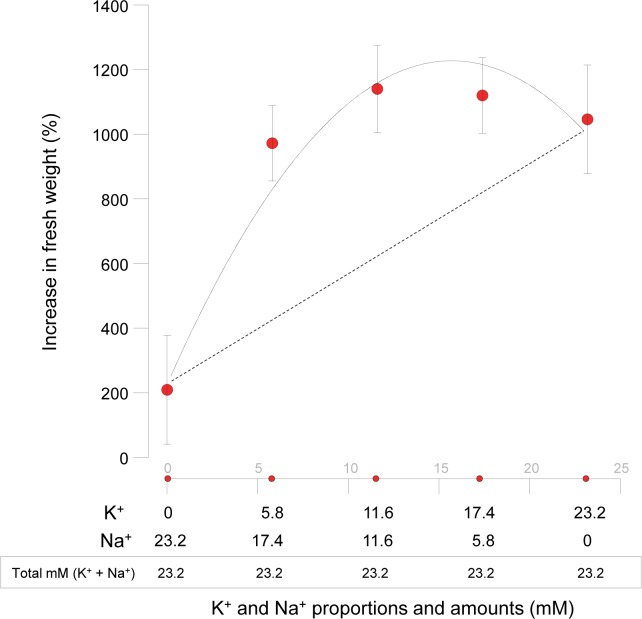
The plotted response surface shows a sharp reduction in fresh weight at 0 mM K+ / 23.2 mM Na+ (Fig 2), revealing that for in vitro growth of this tissue Na+ cannot completely substitute for K+, a result consistent with the view that Na+ can only substitute for K+ for certain nonspecific functions [22]. From 5.9 mM K+ / 17.3 mM Na+ to 23 mM K+ / 0.2 Na+ the response is essentially flat, indicating 1) Na+ can partially substitute for K+ and 2) a threshold where Na+ cannot completely substitute for K+.
Fig 2. Response plot of the effect of the 2-component K+ and Na+ mixture on % increase in fresh weight of Valencia sweet orange nonembryogenic callus.
The significant linear component, depicted by the dotted line, is the estimated response at each vertex (251% and 1011%), and the significant quadratic component indicates nonlinear blending or that the response deviates (above) from what would be predicted by the linear component. However, the graphic reveals a sharp reduction in fresh weight at 0 mM K+ / 23.2 mM Na+ and essentially a flat relationship between 5.9 mM K+ / 17.3 mM Na+ to 23 mM K+ / 0.2 Na+. This indicates a threshold where Na+ cannot completely substitute for K+.
Discussion
ARS-Media for Excel addresses two common problems in experimental biology. The first is how to exactly formulate ionic solutions of ecological/biological relevance. These types of solutions are relevant to virtually every aspect of biological and agricultural science, from plant mineral nutrition to ecological compositions in nature to the ionic makeup of spinal fluid. The study by Angle et al. [4] and their precise and extensive description of their attempt to construct a recipe to replicate the ionic composition of a soil solution representative of a loamy agricultural soil with a neutral pH illustrates the difficulty of solving, or attempting to solve, this type of problem by hand. ARS-Media for Excel solves this problem.
The second problem is how to design an experiment that can determine ion-specific effects. ARS-Media for Excel was used to determine the ion-specific effects of K+ and Na+ on the growth of a sweet orange nonembryogenic cell line. The conclusions were similar to what others have concluded about the relationship between K+ and Na+. Wakeel et al. (2015) [22] in their review of the substitution of potassium (K+) by sodium (Na+) explain that K+ is “the only monovalent cation which is essential for all higher plants”. However, for certain nonspecific metabolic functions, such as osmoregulation, Na+ can substitute for K+. Understanding the substitution of K+ with Na+ is of practical importance as a potential tool to address the increasing problem of soil salinity, crop production, and the differential nutritional needs of plants and animals for Na+ [22]. However, experiments to determine the effects of K+ with Na+ on plant growth are generally confounded. To the best of my knowledge, this seemingly simple experiment represents the only example of an experiment free of ion-confounding to quantify the effects of K+ and Na+ that are free of the confounding influence of co-varied ions. Designing experiments free of ion confounding should either confirm and/or clarify current knowledge of an ion’s effects, or reveal new and possibly unexpected effects, particularly those related to interactions, synergies, and antagonisms.
The two problems are not mutually exclusive but are closely related. After formulating an ecological/biological ionic solution the next logical step is to determine the ions in the solution that have the largest effects on an organism’s growth. One example is to first formulate a water body where toxic algae blooms regularly occur followed by ion-based designs determine the extent to which mineral nutrients affect the growth of toxic algae species. A second example is developing in vitro growth media based on the ionic composition of a “healthy” cell/tissue/organ/plant. Ion-based experiments would use the ionic composition to construct and orient the design space.
Calculating salt recipes without using the linear programming algorithm described by Eq (1) is extremely difficult and, for practical purposes, not possible, and salt recipes generated “by hand” are only rough approximations of the target ion levels [4]. ARS-Media for Excel makes these calculations trivial, thus making these types of experiments possible. Designing experiments based on ions is almost nonexistent in the literature but is necessary to understanding what the ions effects, and more importantly, their interactions with other ions. This type of information would complement well the current advances in molecular biology and omics.
Acknowledgments
I thank Mr. Eldridge Wynn for his careful preparation of the media formulations, growth of the plant cultures, and setup and collection of the data for this study. Per USDA, ARS Policies & Procedures for publishing with non-USDA publishers–“Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.” “USDA is an equal opportunity provider and employer.”
Data Availability
Relevant data are within the paper and ARS-Media for Excel spreadsheet available from the USDA ARS software website—http://www.ars.usda.gov/services/software/software.htm.
Funding Statement
The author received no specific funding for this work.
References
- 1.Smith EJ, Davison W, Hamilton-Taylor J. Methods for preparing synthetic freshwaters. Water Res. 2002;36(5):1286–96. 10.1016/s0043-1354(01)00341-4 [DOI] [PubMed] [Google Scholar]
- 2.Morard P, Henry M. Optimization of the mineral composition of in vitro culture media. Journal of Plant Nutrition. 1998;21(8):1565–76. 10.1080/01904169809365504 [DOI] [Google Scholar]
- 3.Monteiro ACBDA Higashi EN, Gonçalves AN Rodriguez APM. A novel approach for the definition of the inorganic medium components for micropropagation of yellow passionfruit (Passiflora edulis sims. F. Flavicarpa deg.). In Vitro Cellular and Developmental Biology—Plant. 2000;36(6):527–31. [Google Scholar]
- 4.Angle JS, McGrath SP, Chaney RL. New culture medium containing ionic concentrations of nutrients similar to concentrations found in the soil solution. Appl Environ Microbiol. 1991;57(12):3674–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litvay JDJ M. A.; Verma D.; Einspahr D.; Weyrauch K. Conifer Suspension Culture Medium Development Using Analytical Data from Developing Seeds. Inst Paper Chem Tech Paper Ser. 1981;115:1–17. [Google Scholar]
- 6.Nas MN, Read PE. A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic. 2004;101(1–2):189–200. 10.1016/j.scienta.2003.10.004 [DOI] [Google Scholar]
- 7.Gonçalves S, Correia PJ, Martins-Loução MA, Romano A. A new medium formulation for in vitro rooting of carob tree based on leaf macronutrients concentrations. Biol Plant. 2005;49(2):277–80. 10.1007/s10535-005-7280-4 [DOI] [Google Scholar]
- 8.Staikidou I, Selby C, Hanks GR. Development of a medium for in vitro culture of Galanthus species based on the mineral composition of bulbs. Journal of Horticultural Science and Biotechnology. 2006;81(3):537–45. [Google Scholar]
- 9.Ashrafi EN, Vahdati K, Ebrahimzadeh H, Mirmasoumi M. Analysis of in-vitro explants mineral contents to modify medium mineral composition for enhancing growth of Persian walnut (Juglans regia L.). Journal of Food, Agriculture and Environment. 2010;8(2):325–9. [Google Scholar]
- 10.Nas MN, Bölek Y, Sevgin N. Shortcut to long-distance developing of a tissue culture medium: micropropagation of mature almond cultivars as a case study. Turkish Journal of Botany. 2013;37:1134–44. 10.3906/bot-1302-49 [DOI] [Google Scholar]
- 11.Oberschelp GPJ, Gonçalves AN, Meneghetti EC, Graner ÉM, de Almeida M. Eucalyptus dunnii Maiden plant regeneration via shoot organogenesis on a new basal medium based on the mineral composition of young stump shoots. In Vitro Cell Dev Biol Plant. 2015;51(6):626–36. 10.1007/s11627-015-9715-8 [DOI] [Google Scholar]
- 12.Niedz RP, Evens TJ. A solution to the problem of ion confounding in experimental biology. Nat Methods. 2006;3(6):417 10.1038/nmeth0606-417 [DOI] [PubMed] [Google Scholar]
- 13.Evens TJ, Niedz RP. Are Hofmeister series relevant to modern ion-specific effects research? Scholarly Research Exchange. 2008. 10.3814/2008/761829 [DOI] [Google Scholar]
- 14.Krishnasamy K, Bell R, Ma Q. Wheat responses to sodium vary with potassium use efficiency of cultivars. Frontiers in plant science. 2014;5:631 10.3389/fpls.2014.00631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erel R, Yermiyahu U, Ben-Gal A, Dag A, Shapira O, Schwartz A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J Plant Physiol. 2015;177:1–10. 10.1016/j.jplph.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 16.Erel R, Ben-Gal A, Dag A, Schwartz A, Yermiyahu U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiology. 2014;34(10):1102–17. 10.1093/treephys/tpu081 [DOI] [PubMed] [Google Scholar]
- 17.Schecher WD, McAvoy DC. MINEQL+ A Chemical Equilibrium Modeling System: Version 4.5 for Windows User's Manual. Hallowell, Maine: Environmental Research Software; 2003. [Google Scholar]
- 18.Niedz RP, Evens TJ. Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol Plant. 2007;43(4):370–81. 10.1007/s11627-007-9062-5 [DOI] [Google Scholar]
- 19.Murashige T, Tucker DPH. Growth factor requirements of citrus tissue culture. Proc First Intl Citrus Symp. 1969;3:1155–61. [Google Scholar]
- 20.Murashige T, Skoog F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol Plant. 1962;15(3):473–97. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 21.Anderson MJ, Whitcomb PJ. RSM simplified: optimizing processes using response surface methods for design of experiments New York, NY: Productivity Press; 2005. [Google Scholar]
- 22.Wakeel A, Farooq M, Qadir M, Schubert S. Potassium Substitution by Sodium in Plants. Crit Rev Plant Sci. 2011;30(4):401–13. 10.1080/07352689.2011.587728 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data are within the paper and ARS-Media for Excel spreadsheet available from the USDA ARS software website—http://www.ars.usda.gov/services/software/software.htm.