Abstract
Background
Obesity has become a major global health challenge due to its increasing prevalence, and the associated health risk. It is the main cause of various metabolic diseases including diabetes, hypertension, cardiovascular disease, stroke and certain forms of cancer.
Methods and Results
In the present study we evaluated the anti-obesity property of Daesiho-tang (DSHT), an herbal medicine, using high fat diet (HFD)-induced obese mice as a model. Our results showed that DSHT ameliorated body weight gain, decreased total body fat, regulated expression of leptin and adiponectin genes of adipose tissue and exerted an anti-diabetic effect by attenuating fasting glucose level and serum insulin level in HFD-fed animals. In addition, DSHT-treatment significantly reduced total cholesterol (TC), triglycerides (TG) and increased high density lipoprotein-cholesterol (HDL), glutamic pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) levels in serum and reduced deposition of fat droplets in liver. DSHT treatment resulted in significantly increased relative abundance of bacteria including Bacteroidetes, Bacteroidetes/Firmicutes ratio, Akkermansia Bifidobacterium., Lactobacillus, and decreased the level of Firmicutes. Using RT2 profiler PCR array, 39 (46%) genes were found to be differentially expressed in HFD-fed mice compared to normal control. However, normal gene expressions were restored in 36 (92%) genes of HFD-fed mice, when co-exposed to DSHT.
Conclusion/Major Findings
The results of this study demonstrated that DSHT is an effective herbal formulation in attenuation of obesity in HFD-fed mice through alteration of gene expressions and modulation of intestinal microbiota.
Introduction
Obesity, a common metabolic disease, has now become a major global health challenge due to its increasing prevalence, and the associated health risk [1]. Worldwide, approximately 600 million individuals are suffering from obesity [2] and in USA alone, more than 35% of men and women are obese, which has now outnumbered the total overweight individuals [3]. Obesity is associated with various complication and diseases including diabetes, hypertension, cardiovascular disease, stroke and certain forms of cancer [4].
In obesity, increased proliferation and/or differentiation of adipocyte precursor cells has been reported, which facilitates the accumulation of excessive liver fat and elevates lipid concentrations in blood [5]. Increased adipogenesis accompanied by adipocyte differentiation is regulated by a complex network of genes [6]. Accumulating evidence suggests that the gut microbiota also play an important role in human obesity by influencing energy extraction, inflammation, and neuroendocrine secretions [7]. These microbial communities are predominantly represented by two phyla; Firmicutes (includes Ruminococcus, Clostridium, Eubacterium and Lactobacillus) and Bacteroidetes (includes Prevotella, Bacteroides) with significant numbers of Actinobacteria (Bifidobacterium) and Proteobacteria members [8].
Various drugs including orlistat are used for the treatment and prevention of obesity. Orlistat, is a semisynthetic hydrogenated derivative of lipstatin, a natural compound that is produced by the Actinobacterium Streptomyces toxytricini. This drug is a potent and selective inhibitor of the gastric and pancreatic lipases, the enzymes playing a key role in the digestion of dietary fat [9, 10] The inhibitory action of orlistat is mediated via covalent binding to the serine residue of the active site of the above mentioned enzymes, thus preventing triglyceride digestion and body weight gain [11]. Other anti-obesity drugs either suppress food-intake by regulating the central nervous system, or inhibit the absorption of specific components in the food [12]. However, these anti-obesity drugs cause manifest various side-effects, including gastro-intestinal discomfort, insomnia, headaches, flatulence and diarrhea [12]. Because of the high prevalence of obesity and unavoidable side-effects of the currently available anti-obesity drugs, the development of herbal medicinal products for treatment of obesity has become a global interest.
DSHT, an important herbal formulation in traditional eastern Asian medicine, is known for anti-diabetic [13], anti-hepatotoxic [14], anti-hypertensive and arterial contraction properties [15], protective effect on hypoxic E18 cortical neuroblast [16] DSHT also showed preventive and therapeutic effects against gallstone formation [17]. However, the anti-obesity activity of this herbal medicine has not yet been studied.
The current study was conducted in-order to evaluate the anti-obesity property of DSHT using high fat diet (HFD) fed obese mice as a model. We also investigated the mode of action of this drug, especially emphasizing its impact on the distribution of gut microbiota and influence on expression of genes playing a key role in the cholesterol and lipid metabolism.
Material and Methods
Herbal preparation
The herbal formula DSHT (Daesiho-tang) was procured from Hanpoong pharmaceutical (Seoul, Republic of Korea). It is a combination of eight medicinal herbs; the names and the composition are given in S1 Table. For extraction of DSHT, chopped herbs were mixed with 10 times volume of water and incubated at 100°C for 4 h. After incubation the extract was filtered and freeze dried for 3 days to make dry powder.
Ethical statement
The animals used in this study were housed and cared in accordance to the National Institute of Health (NIH) guidelines for the care and use of laboratory animals [18]. The experimental protocol was approved by Institute Animal Ethical Committee, Dongguk University, Republic of Korea (No: IACUC 201409111). Animals were anesthetized with a combination of Zoletil (tiletamine-zolazepam, Virbac, Carros, France) and Rompun (xylazine-hydrochloride, Bayer, Leverkusen, Germany) (1:1, v/v).
Animal experiments and sample collection
Six-week old, male C57BL/6 mice were purchased from Koatech (Pyeongtaek, Republic of Korea). The animals were acclimatized for 4 weeks prior to the experiments. All animals were kept at a constant temperature of 21°C, relative humidity of 55% and under a 12 h light-dark cycle. The animals were maintained on HFD except those who were segregated to serve as normal control (provided with a normal chow diet). After four weeks, animals were divided into four groups, a normal chow diet group as stated above (n = 7), HFD group (n = 7), and a HFD treated with either DSHT (700 mg/kg/day orally; n = 7; DSHT), or orlistat (10mg/kg body weight orally n = 7). The normal chow diet was comprised of food with 12.7% of the calories being derived from fat. While, the HFD consisted of food with 60% of the calories being contributed by fat. Body weights of animals were measured weekly. The food intake was measured two times per week during experiment period. Food efficiency ratio was calculated as total body weight gain divided by total food intake (g/g) [19]. Mice were maintained on the treatment for 12 weeks, and were then subjected to oral glucose tolerance test following a 16 h fasting as described below. Finally, the animals were sacrificed under anesthesia as stated above. Blood samples were collected before the animals were sacrificed for the measurements of serum biochemical parameters and insulin level. Tissues used for gene expression analysis were removed, immediately transferred into RNAlater RNA stabilization reagent (QIAGEN, Valencia, CA, USA) and stored at -80°C until needed. For histological analysis, the excised tissues were washed rapidly, fixed in 10% neutral buffered formaldehyde and stored until used.
Serum biochemistry analysis
After collection, the blood samples were kept at room temperature for 1h to allow clotting, and then centrifuged for 15 min at 3000 rpm. The serum was separated and stored at -80°C until used. The high density lipoprotein-cholesterol (HDL), triglycerides (TG) and total cholesterol (TC) levels were measured using commercial kits (Asan pharmaceutical, Seoul, Republic of Korea) as per kit manufacturer’s instructions. Serum insulin level in the oral glucose tolerance test was determined by enzyme-linked immunosorbent assay using a commercial kit (Mercodia AB, Uppsala, Sweden) following the kit manufacturer’s protocol.
Oral glucose tolerance test (OGTT)
After the desired treatments, OGTT was performed following a 16 h fasting of the animals. Glucose dissolved in water was administered orally through gavage at a dose of 1 g/kg body weight and OGTT was performed at every 30 min interval (30, 60, 90 and 120 min) after administration of glucose. Blood was collected from the tail vein, serum was separated as described above, and the glucose concentrations of the samples were determined using a blood glucose meter (Accu-Check Advantage, Roche, Charles Avenue, West Sussex, UK).
Histological analysis of liver
Liver tissues fixed in 10% neutral buffered formaldehyde were sliced (5 μm in thickness) and trimmed transversely into serial sections using a cryo-microtome (LEICA Microsystem, Wetzlar, Germany). Sections were subsequently deparaffinized in xylene, rehydrated through an ascending ethanol series and stained with haematoxylin-eosin (HE) for 1 min and then with oil red O solution (Cayman Chemical, Ann Arbor, MI, USA) for 15 min at 60°C. Mounting was performed with xylene-based media. The slides were observed under an Olympus BX 61 microscope (Olympus Tokyo, Japan) at 200X magnification and images were acquired using Olympus DP70 digital camera (Olympus).
Measurement of expression of leptin and adiponectin genes in fat tissue
Quantitative real time-PCR (qRT-PCR) was performed to determine the expression of leptin and adiponectin genes in fat tissue. Briefly, total RNA was isolated from the adipose tissue stored in RNAlater RNA stabilization reagent (QIAGEN) using TRIsure reagent (Bioline, Taunton, MA, USA). The RNA samples were quantified and their purity was checked by measurement of optical densities at 260 and 280 nm. The isolated RNA was reverse transcribed using an AccuPower® RT PreMix kit (Bioneer, Daejeon, Republic of Korea) and oligo dt primers (Invitrogen, Carlsbad, CA, USA) in accordance to the kit manufacturer’s protocol. qPCR reaction was performed in a total volume of 20μL, containing 10μL SYBR Green master mix, 1 μg of cDNA, and gene-specific primers (10 pmol for each, S2 Table) using a Light Cycler instrument (Roche, Indianapolis, ID, USA). A dedicated Light Cycler software (Roche Applied Science) was employed for quantification of relative gene expression as 2-Δct using β-actin as a housekeeping gene (ΔCt = Cttarget gene—Ctβ-actin) [20]. The results are expressed as normalized fold values relative to the control.
Microbial analysis of mouse stool using polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE)
After collection, stools were stored at -80°C until analyzed. Genomic DNA was isolated from the stool using QIAamp stool DNA mini kit (QIAGEN) in accordance to the kit manufacturer’s instructions. The purity and concentration of DNA in the samples were determined using a NanoDrop™ (Thermo scientific, Wilmington, DE, USA), and the quality of DNA was further assessed by gel-electrophoresis. The extracted DNA was PCR amplified using universal primers for the bacterial 16S rRNA gene (S2 Table). The first PCR products were separated on 1% agarose gel, stained with ethidium bromide (Etbr, Bio-Rad, Hercules, CA, USA) and 1.5 kb DNA bands were gel-eluted using an Accuprep gel purification kit (QIAGEN). The second round of PCR amplification was performed for the V3 region from 16S rDNA using the primers 314f-GC and 518r (S2 Table) as previously described [17]. DGGE of the second PCR products was carried out using a D Code universal mutation detection system (Bio-Rad, Hercules CA, USA). Briefly, 15 μL of the samples were loaded on 40% acrylamide/bis (37.5:1 v/v) gels with denaturing gradient maintained at 30–60% (where 100% represents 7 M urea in combination with 40% (v/v) formamide) in 1xTAE buffer (ViroMed, Seoul, Republic of Korea). Electrophoresis was performed at 80 V and at a temperature of 60°C for 13 h. After staining with Etbr at room temperature for 20 min, the gels were photographed under UV illumination (LAS-3000; Fuji photo film, Tokyo, Japan). Analysis of the band patterns in the images was carried out using Bionumerics 3.0 software (Applied Maths, Sint-Martens-Latem, Belgium). Data were analyzed using an Unweighted Pair-Group Method with Arithmetic mean (UPGMA) clustering procedure based on genetic similarity expressed by the Jaccard coefficient. Principal component analysis (PCA) was also performed on the DGGE profiles.
Quantification of bacterial abundance in stool samples using qRT-PCR
qRT-PCR of the stool DNA samples was performed in a total reaction mixture volume of 20 μl as described above for qRT-PCR analysis of fat tissue leptin and adiponectin genes. The primers for the bacterial 16S rRNA genes used in this study are shown in S2 Table. The annealing temperature, concentrations of DNA and primers were optimized as previously described [20]. Analyses of the data and determination of ct value were carried out using Light Cycler software (Roche Applied Science). The relative quantification of bacteria DNA was performed using 2-Δct method, and the normalized fold values relative to the normal group were calculated.
Gene expression profiling of adipose tissue by RT2-PCR array
Total RNA was isolated from adipose tissues stored in RNAlater RNA stabilization reagent (QIAGEN) using TRIZOL (Invitrogen) according to the manufacturer’s instructions. The extracted RNA was reversed transcribed using a similar method as described above for qRT-PCR analysis of fat tissue leptin and adiponectin genes. Expression of genes involved in lipid and cholesterol metabolism was analyzed by PCR array using a 96-well Human Lipoprotein Signaling & Cholesterol Metabolism RT2 Profiler PCR Array Kit (PAMM-080ZC-2, SABiosciences, Valencia, CA, USA) and a LightCycler 480 PCR system (Roche, Basel, Switzerland) according to the kit manufacturer's instructions. The data were analyzed using SDS Software 2.3 and web-based programs at www.SABiosciences.com/pcrarraydataanalysis.php (SABiosciences). Gene expression was normalized to the mean of all house-keeping genes in the array. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the differentially expressed genes were determined using STRING version 10 [21].
Confirmative gene expression study using qRT-PCR
Seven differentially expressed genes in the RT2 array were further verified using qRT-PCR. The analysis was performed as described above for the qRT-PCR of fat tissue leptin and adiponectin genes using the primers shown in S2 Table.
Statistical analyses
The values are expressed as mean ± SD. The statistical package for social science (SPSS) software program (version 17.0; SPSS, Chicago, IL, USA) was applied for data analyses. The mean value differences were assessed using Student’s t-test, and the statistical significance was set at a p-value of less than 0.05.
Results
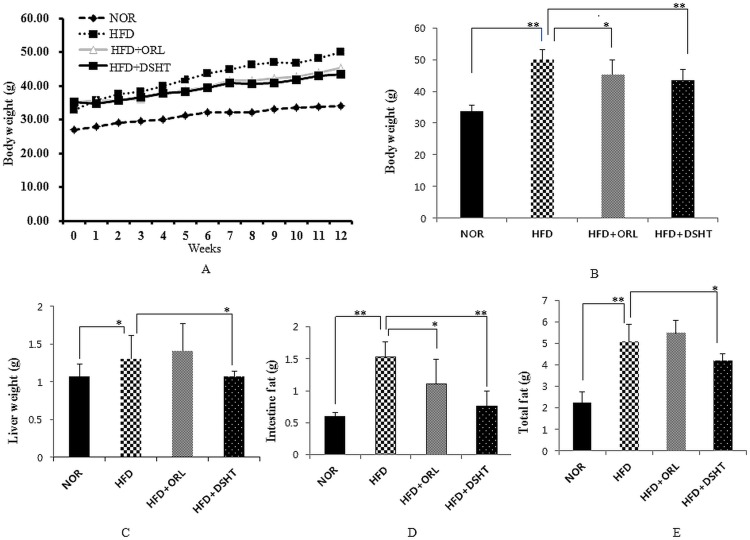
DSHT treatment attenuated total body weight, liver weight, total and intestinal fat in HFD-induced obese mice
Total body weight measured weekly increased at a higher rate in HFD-fed animals, while the body weight of HFD + DSHT-treated animals increased at a lower rate (Fig 1A). At the end of 12th week, the body weight of HFD-fed mice was significantly (p<0.05) higher as compared to that of animals in normal group (NOR), indicating an effective HFD-induced obesity in the mice. However, an exposure of HFD-fed animals to DSHT significantly (p<0.005) attenuated total body weight gain (Fig 1B). Similar results were shown by the positive control group (HFD + ORL). The FER was also significantly higher in HFD-fed mice as compared with NOR group. However, this parameter in HFD-fed animals decreased significantly when treated with either DSHT or ORL (S3 Table).
Fig 1. Effect of DSHT on total body weight (gain), liver weight, intestinal and total fat of HFD-fed C57BL/6 mice.
(A) Total body weight of normal (NOR), high fat diet (HFD), orilistat (ORL) or daesiho-tang (DSHT) fed animals measured weekly. (B) The total body weight of NOR, HFD, HFD+ORL and HFD+DSHT fed animals after 12 weeks. (C) The liver weight of NOR, HFD, HFD+ORL and HFD+DSHT diet fed animals. (D) Intestinal fat of NOR, HFD, HFD+ORL and HFD+DSHT fed animals. (E) The total fat of NOR, HFD, HFD+ORL and HFD+DSHT fed animals. Data were represented as mean ± SD (n = 7). The results were compared by Student’s t-test (** corresponds to p<0.005, * corresponds to p<0.05). The error bars represent the SD.
The liver weight, intestinal fat, and total fat were also significantly (p<0.05) augmented in HFD-fed animals compared to that of NOR group. However, DSHT co-treatment significantly (p<0.05) attenuated the liver weight, intestinal fat, and total fat in the HFD-fed mice (Fig 1C–1E). In contrast, co-exposure of HFD-fed animals to ORL caused a significant (p<0.05) reduction in intestinal fat, but did not produce any significant effect on liver weight and total fat. Collectively, our results indicate that DSHT can effectively prevent HFD-induced body weight gain and fat deposition.
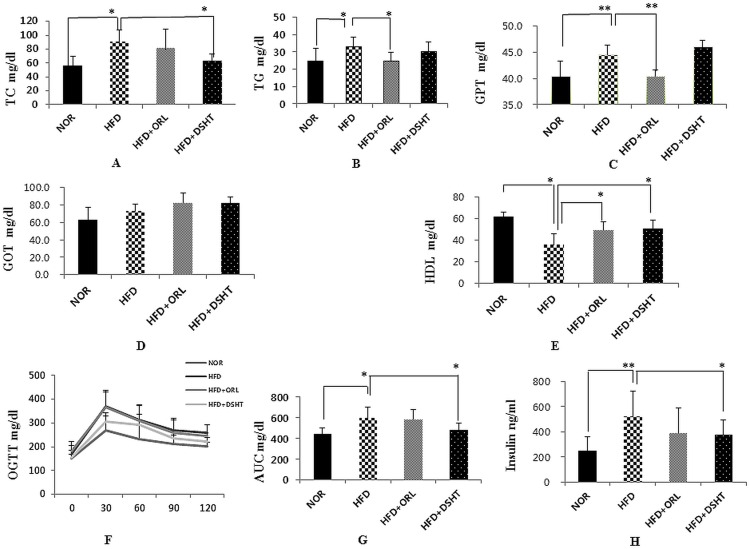
Effect of DSHT on serum biochemical parameters
In comparison to NOR, HFD-fed mice showed significantly (p<0.05) higher levels of serum TC, TG, and GPT and significantly (p<0.05) lower content of serum HDL (Fig 2A–2E). However, treatment of HFD-fed mice with DSHT, but not ORL, significantly (p<0.05) attenuated the level of serum TC. The serum TG content was insignificantly and significantly (p<0.05) reduced in HFD+DSHT- and HFD+ORL-treated animals, respectively vs. NOR group. The HDL level was significantly (p<0.05) higher in both HFD+DSHT- and HFD+ORL-treated mice vs. HFD-fed animals No significant differences in the levels of serum GOT and GPT were found between the HFD and HFD + DSHT groups, while HFD + ORL-treated mice showed significantly lower level of serum GPT vs. HFD-fed animals
Fig 2. Effect of DSHT on biochemical parameter, glucose homeostasis, and insulin resistance in HFD-fed C57BL/6 mice.
(A) The TC levels of normal (NOR), high fat diet (HFD), orilistat (ORL), and daesiho-tang (DSHT) treated animals after 12 weeks. (B) The TG levels of NOR, HFD, HFD+ORL and HFD+DSHT treated animals. (C) The GPT levels of NOR, HFD, HFD+ORL and HFD+DSHT treated animals. (D) The GOT levels of NOR, HFD, HFD+ORL and HFD+DSHT treated animals. (E) The HDL levels of NOR, HFD, HFD+ORL and HFD+DSHT treated animals. (F) Oral Glucose Tolerance test (OGTT) of normal (NOR), high fat diet (HFD), orilistat (ORL) and daesiho-tang (DSHT) fed animals after 12 weeks. (G) Area under curve of NOR, HFD, HFD+ORL and HFD+DSHT fed animals. (H) Fasting insulin of NOR, HFD, HFD+ORL and HFD+DSHT fed animals. Data were represented as mean ± SD (n = 7). The results were compared by Student’s t-test (** corresponds to p<0.005, * corresponds to p<0.05). The error bars represent the SD.
DSHT ameliorated glucose homeostasis and insulin resistance
As expected, higher serum glucose levels were observed in HFD-fed animals compared to that of NOR group at all-time points of OGTT (30, 60, 90 and 120 min) (Fig 2F). In parallel, the area under the curve (AUC) of the blood glucose response was also found to be significantly higher in HFD group compared to NOR. However, the animals of HFD + DSHT group, but not HFD + ORL group, showed significantly (p<0.05) lower serum glucose levels at all-time points of OGTT (30–120 min) and AUC of the glucose response compared to that HFD group (Fig 2F & 2G). Following the termination of the OGTT, the serum insulin level was also found to be significantly (p<0.05) elevated in HFD group compared to NOR. However, the serum insulin level was significantly (p<0.05) attenuated in HFD-fed animals when treated with DSHT, but not ORL (Fig 2H)
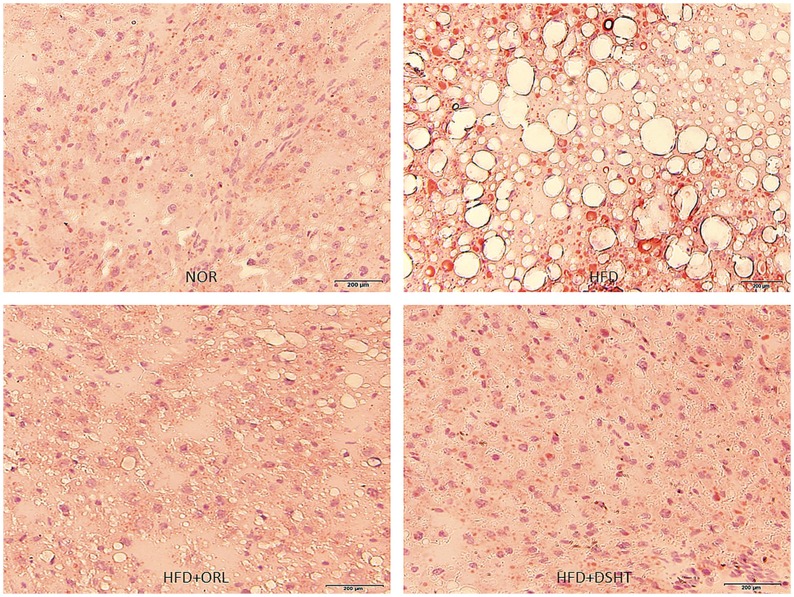
DSHT ameliorated HFD-induced histological alterations and fat deposition in liver
Histopathological evaluation of the liver tissue accompanied with oil red O staining demonstrated normal lobular architecture with the appearance of only a few small-sized fat droplets in untreated animals (Fig 3A). In contrast, an aberrant histological structure dominated by substantial deposition of fat in the form of large-sized vacuoles was observed in the liver of HFD-fed animals, indicating the state of fatty liver (Fig 3B). However, the architecture of liver tissue appeared to be normal with minimal or even without deposition of fat droplets in HFD-fed animals treated with DSHT (Fig 3D).a profile quite similar to that shown by ORL (Fig 3C).
Fig 3. Effect of DSHT on HFD-induced histopathological changes of liver tissue stained with oil red O staining.
(A) Representative image of liver tissue of normal control animal (NOR) stained with Oil red O. (B) Representative image of liver tissue of animals treated with HFD. (C) Representative image of liver tissue of animals treated HFD+ORL. (D) Representative image of liver tissue of HFD+DSHT treated animals. Pathophysiological examination of the tissue sections was performed under light microscopy with 200x magnification.
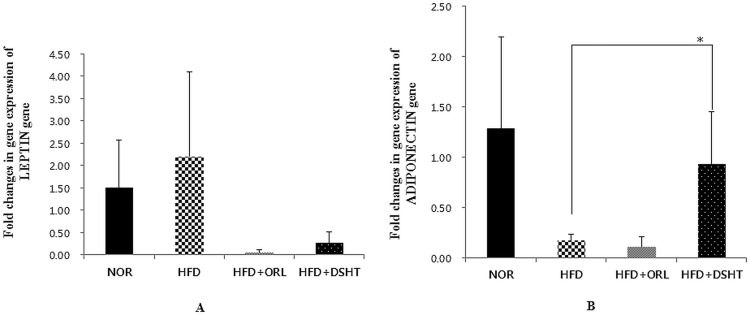
Effect of DSHT on expressions of leptin and adiponectin genes in fat tissues
To evaluate the changes in gene expression in fat tissues in HFD+DSHT-treated animals, the expression of leptin and adiponectin genes were quantified using real-time RT-PCR. The leptin gene was up-regulated and adiponectin gene was down-regulated in HFD-fed animals compared to NOR (Fig 4). However, DSHT treatment resulted in down-regulation of leptin gene and significant (p<0.05) up-regulation of adiponectin gene in HFD-fed mice. In positive control (HFD + ORL group), also a similar kind of profile was observed for leptin gene expression, however, no increase in adiponectin gene expression was noticed
Fig 4. Effect of DSHT on leptin and adiponectin gene expressions in HFD-fed C57BL/6j mice.
(A) Folds changes in leptin gene expression in normal (NOR), high fat diet (HFD), orilistat (ORL), and daesiho-tang (DSHT) fed animals after 12 weeks. (B) adiponectin gene expression in NOR, HFD, HFD+ORL and HFD+DSHT treated animals. Data were represented as mean ± SD (n = 4). The results were compared by Student’s t-test (* corresponds to p<0.05). The error bars represent the SD.
DSHT altered gut microbial distribution
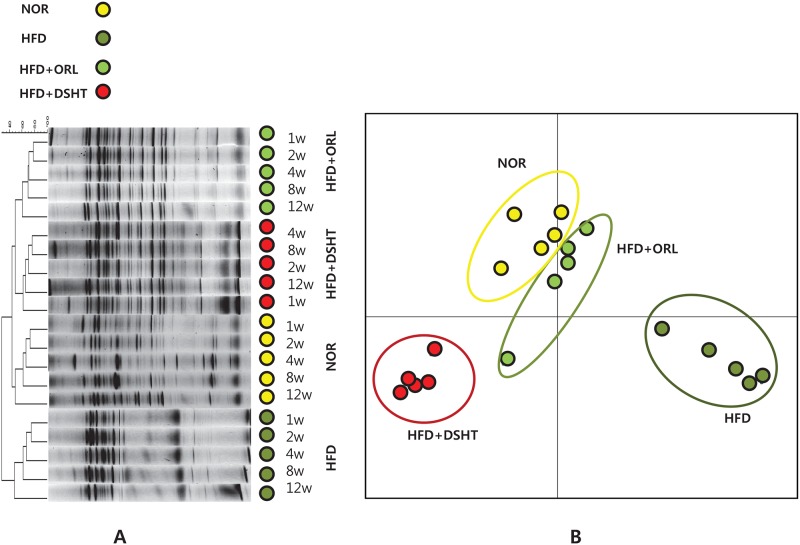
HFD Feeding changed the gut floral distribution markedly, which was further altered upon treatment with DSHT or ORL (Fig 5A). The banding patterns obtained in DGGE demonstrated similarities among HFD + DSHT, HFD + ORL, and NOR groups. The PCA analysis also revealed notable differences between the microbial communities of HFD- and HFD + DSHT- treated mice. More specifically, the microbial distribution of animals treated with HFD + DSHT was closely related to that shown by HFD + ORL group (Fig 5B).
Fig 5. PCR-denaturing gradient gel electrophoresis (DGGE) fingerprinting, and PCA analysis of different microbial flora in mice stool.
(A) Banding patters and clustering obtained from PCR-DGGE fingerprinting of mice stool DNA of Normal, HFD, ORL and DSHT. (B) PCA analysis of PCR-DGGE data based on distance matrix showing the similarity between bacterial communities.
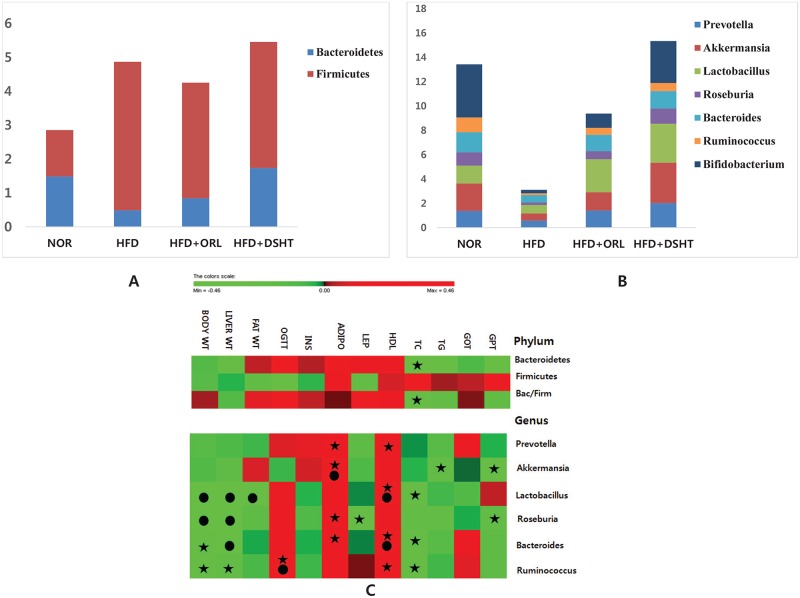
In addition, the population of several gut floral strain, were evaluated using quantitative real time-PCR (Fig 6A and 6B). The relative abundances of phylum Bacteroidetes and genus Prevotella, Lactobacillus, Roseburia, Bacteroides, Ruminococcus, and Bifidobacterium were found to be significantly (p<0.05) lower in HFD-fed animals compared to NOR group. In contrast, the load of phylum Firmicutes was significantly higher in HFD group vs. NOR group. However, the relative abundances of Roseburia, Bacteroides, Ruminococcus, and Bifidobacterium in HFD group were significantly (p<0.05) increased upon treatment with either DSHT or ORL. While the exposure of HFD-fed mice to DSHT and ORL caused significant and insignificant increase in Lactobacillus, respectively, and insignificant and significant increase in Prevotella, respectively. On the other hand, the relative abundance of Bacteroidetes in HFD group was significantly (p<0.05) and insignificantly but prominently increased in response to the treatments with ORL and DSHT, respectively. In contrast, the relative abundance of Firmicutes in HFD-fed animals was insignificantly decreased when exposed to DSHT or ORL. The Bacteroidetes/Firmicutes ratio was insignificantly lower in HFD group vs. NOR group, but increased in both HFD+DSHT and HFD+ORL groups. There was an insignificant, but prominent, decrease in the relative abundance of Akkermansia in mice in response to HFD feeding and exposure of the HFD-fed animals to either DSHT or ORL resulted in an insignificant, but definite increase in this bacterial population.
Fig 6. Relative abundances of different microbial flora in mice stool, and their correlations with vital metabolic parameters linked to obesity and liver functions using head-map.
(A) The relative abundance of different gut flora was quantified by q-RT-PCR in animals fed on normal diet, High fat diet, HFD+ORL and HFD+DSHT. The relative abundance of Phylum Bacteroidetes decrease and Firmicutes were increased in animals treated with HFD alone. (B) The relative abundance of Genus Prevotella, Akkermansia, Lactobacillus, Roseburia, Bacteroides, Ruminococcus and Bifidobacterium were decreased in animals treated with HFD alone. However, the relative abundance of these bacteria was restored to normal in animals treated with DSHT or ORL. (C) Correlations between relative abundance of the gut microbial community and vital metabolic parameters linked to obesity as well as parameters related to liver functions are shown by the heat map. (A symbol of ★ indicates absolute value of Pearson r.>.4; a symbol of ● indicates statistical significance of P<0.05.).
Correlations between relative abundances of the gut microbial communities and vital metabolic parameters linked to obesity as well as parameters related to liver functions are shown by the heat map (Fig 6C). Correlations at each microbial level were determined using two-tailed Pearson’s correlation analysis. At the phylum level, no significant correlation was found between the parameters tested and the relative abundances of Firmicutes or Bacteroidetes or Bacteroidetes/ Firmicutes ratio. However, both Bacteroidetes and Bacteroidetes/ Firmicutes ratio exhibited a definite negative correlation with TC (r = -0.456 and -0.401, respectively). While at the genus level, Lactobacillus and Roseburia demonstrated significant (p<0.05) negative correlations with the body and liver weights. Lactobacillus also showed significant (p<0.05) negative and positive correlations with the total body fat and serum HDL level, respectively, and an insignificant, but obvious negative correlation with serum TC level (r = -0.433). Roseburia on the other hand, exhibited certain positive correlation with the gene expression of adiponectin in adipose tissue (r = 0.490). Prevotella demonstrated insignificant, but definite positive correlations with both the adipose adiponectin gene expression (r = 0.420) and serum HDL level (r = 0.457). A significant (p<0.05) positive correlation was also found between the adipose adiponectin gene expression and the relative abundance of Akkermansia. This genus also showed an insignificant, but a clear negative correlation with serum TG level (r = -0.404). On the other hand, Ruminococcus demonstrated a significant (p<0.05) and an insignificant, but obvious (r = 0.493) positive correlations with OGTT and serum HDL level, respectively. This genus also showed insignificant, but prominent negative correlations with body weight (r = -0.475), liver weight (r = -0.422), and serum TC level (r = -0.464). While Bacteroides exhibited a significant (p<0.05) negative correlation with liver weight and insignificant, but definite negative correlations with body weight (r = -0.441) and serum TC level (r = -0.456). This genus also showed insignificant but certain positive correlations with adipose adiponectin gene expression (r = 0.455) and serum HDL level (r = 0.511).
Effect of DSHT on cholesterol and lipid metabolism protein encoding gene expression of C57BL obese mice
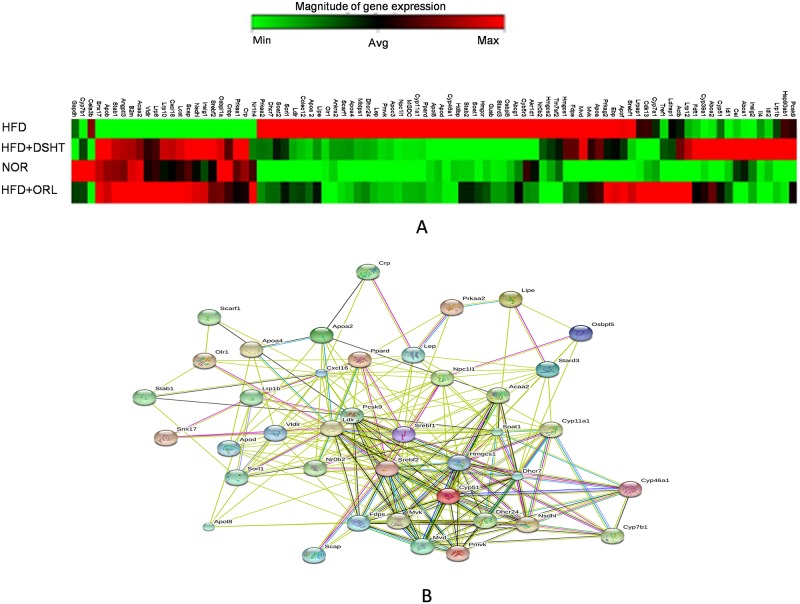
Expression of genes playing a key role in cholesterol and lipid metabolism was evaluated in the HFD, HFD + ORL and HFD + DSHT groups using RT2 PCR array (Fig 7A). The study revealed that expression of 39 genes was significantly differentially expressed (>2 folds) in HFD-fed animals compared to NOR group. Among them, 29 genes were significantly up-regulated by more than 2 folds, and 10 genes were significantly down-regulated by >2 (0.5) folds (Tables 1 and 2). The GO and KEGG pathway analysis indicated that the genes involved in non-alcoholic fatty liver disease (NAFLD), fat digestion and absorption, insulin signaling pathway, PPAR signaling pathway, AMPK signaling pathway, metabolic pathway, steroid biosynthesis and terpenoid back-bone biosynthesis were up-regulated. And the genes involved in low-protein particle and low-density lipoprotein binding and receptor activity were down-expressed (Fig 7B). More specifically, the genes encoding apolipoproteins (apoa2, apoa4, apod, and apol8) and cytochrome P450 family proteins (cyp11a1, cyp461a and cyp51) were up-regulated (>2 folds) in HFD-fed animals compared to NOR, however, expression of these genes in HFD + DSHT-treated animals were decreased compared to HFD group or unchanged vs. NOR. Furthermore except for the cyp51, lrp1b and pcsk9 genes, the gene expression patterns in HFD +DSHT-treated mice were similar to those shown by HFD + ORL group. Interestingly, the polytopic transmembrane protein encoding gene Niemann-Pick C1-Like 1 (npc1l1), which mediates extracellular sterol transport across the brush border membrane was up-regulated by 10 folds in HFD-fed animals vs. NOR. In contrast, the expression of npc1l1 was 1 and 1.2 folds (decreased or unchanged) in animals treated with either HFD + DSHT or HFD + ORL, respectively.
Fig 7. Effect of DSHT on expressions of lipid and cholesterol metabolism genes in HFD-fed c57bl/6j mice.
(A) Hierarchical clustering of gene expressions of mouse fed on HFD, DSHT, ORL and NOR detected by RT2 PCR array. The dendrogram at the left provides a measure of relatedness of gene expression in each group. (B) The interconnected pathway of genes differentially expressed in RT2 PCR array.
Table 1. The expression profiles of genes in HFD, HFD + DSHT, or HFD + ORL-treated mice which were significantly up-regulated in HFD-fed animals by 2 folds.
| Gene name | Protein | HFD | DSHT | ORL |
|---|---|---|---|---|
| Apoa2 | Apolipoprotein A-II | 2.3784 | 1.1173 | 1.4208 |
| Apoa4 | Apolipoprotein A-IV | 3.7842 | 1.5692 | 1.2541 |
| Apod | Apolipoprotein D | 3.5554 | 0.6783 | 0.9571 |
| Apol8 | Apolipoprotein L 8 | 4.084 | 0.8066 | 1.0994 |
| Cyp11a1 | Cytochrome P450, family 11, subfamily a, polypeptide 1 | 6.5432 | 1.2483 | 1.7613 |
| Cyp46a1 | Cytochrome P450, family 46, subfamily a, polypeptide 1 | 6.1475 | 0.9461 | 1.0767 |
| Cyp51 | Cytochrome P450, family 51 | 2 | 4.5631 | 2.6882 |
| Dhcr24 | 24-dehydrocholesterol reductase | 2.514 | 1.2397 | 1.3535 |
| Dhcr7 | 7-dehydrocholesterol reductase | 4.9246 | 2.042 | 2.1535 |
| Fdps | Farnesyl diphosphate synthetase | 4.7899 | 3.605 | 1.9543 |
| Hmgcs1 | 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 | 8.6939 | 6.2767 | 3.1529 |
| Ldlr | Low density lipoprotein receptor | 4.0278 | 1.4948 | 2.0658 |
| Lep | Leptin | 6.5432 | 1.7777 | 1.7613 |
| Lipe | Lipase, hormone sensitive | 2.5315 | 1.3104 | 1.7013 |
| Lrp1b | Low density lipoprotein-related protein 1B (deleted in tumors) | 3.1602 | 6.9163 | 1.5547 |
| Mvd | Mevalonate (diphospho) decarboxylase | 5.0982 | 4.9588 | 2.7638 |
| Mvk | Mevalonate kinase | 3.5064 | 2.4794 | 2.4566 |
| Npc1l1 | NPC1-like 1 | 10.9283 | 1.2483 | 1.7613 |
| Nr0b2 | Nuclear receptor subfamily 0, group B, member 2 | 1.9185 | 1.257 | 0.9374 |
| Olr1 | Oxidized low density lipoprotein (lectin-like) receptor 1 | 5.9381 | 1.2397 | 0.8868 |
| Osbpl5 | Oxysterol binding protein-like 5 | 4.3469 | 0.5824 | 1.5655 |
| Pcsk9 | Proprotein convertase subtilisin/kexin type 9 | 2.2346 | 3.0951 | 2.1238 |
| Pmvk | Phosphomevalonate kinase | 3.249 | 1.1567 | 1.2368 |
| Ppard | Peroxisome proliferator activator receptor delta | 3.8106 | 1.1487 | 1.4306 |
| Prkaa2 | Protein kinase, AMP-activated, alpha 2 catalytic subunit | 2.0279 | 1.2834 | 1.2198 |
| Scarf1 | Scavenger receptor class F, member 1 | 2.1287 | 1.2226 | 1.0693 |
| Soat1 | Sterol O-acyltransferase 1 | 1.9319 | 0.9138 | 1.3629 |
| Sorl1 | Sortilin-related receptor, LDLR class A repeats-containing | 2.2501 | 1.4948 | 1.5764 |
| Srebf1 | Sterol regulatory element binding transcription factor 1 | 2.0279 | 1.0943 | 1.9408 |
| Stard3 | START domain containing 3 | 2.114 | 0.9526 | 1.1783 |
Table 2. The expression profiles of genes in HFD, HFD + DSHT, or HFD + ORL-treated mice which were significantly down-regulated in HFD-fed animals by 2 (0.5) folds.
| Gene name | Protein | HFD | DSHT | ORL |
|---|---|---|---|---|
| Acaa2 | Acetyl-Coenzyme A acyltransferase 2 (mitochondrial 3-oxoacyl-Coenzyme A thiolase) | 0.5905 | 0.9138 | 1.0116 |
| Crp | C-reactive protein, pentraxin-related | 0.4698 | 1.1329 | 0.8332 |
| Cxcl16 | Chemokine (C-X-C motif) ligand 16 | 0.5905 | 1.3104 | 1.4109 |
| Cyp7b1 | Cytochrome P450, family 7, subfamily b, polypeptide 1 | 0.3143 | 0.5625 | 0.5651 |
| Nsdhl | NAD(P) dependent steroid dehydrogenase-like | 0.3686 | 1.6818 | 1.6096 |
| Scap | SREBF chaperone | 0.1582 | 1.434 | 1.4109 |
| Snx17 | Sorting nexin 17 | 0.0272 | 1.1329 | 1.0994 |
| Srebf2 | Sterol regulatory element binding factor 2 | 0.0188 | 1.9588 | 1.4012 |
| Stab1 | Stabilin 1 | 0.2588 | 1.1019 | 1.2114 |
| Vldlr | Very low density lipoprotein receptor | 0.028 | 1.0792 | 1.8234 |
While, the vldlr, stab1, srebf2, snx17, scap, and nsdh1 genes were down-regulated in HFD-fed animals compared to NOR. However, these genes were significantly up-regulated and/ or maintained a normal gene expression in HFD + DSHT or HFD + ORL-treated animals (Table 2).
Confirmative Gene expression study using Real-time RT-PCR
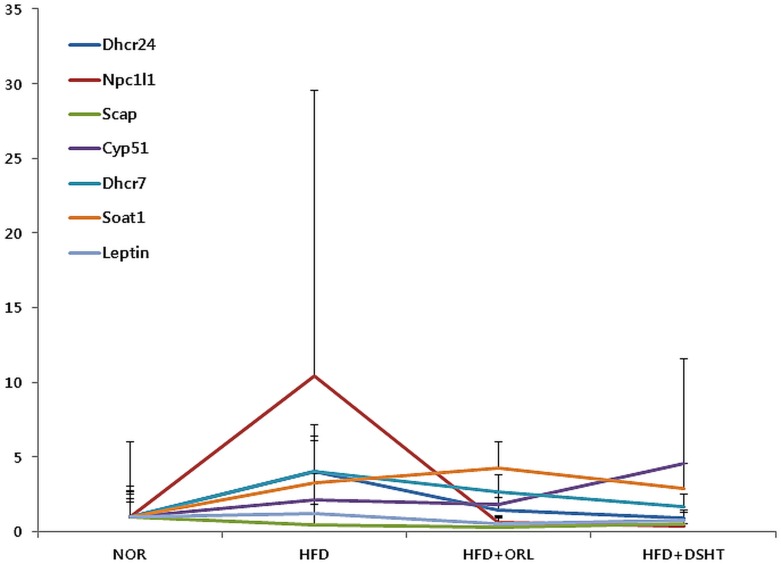
Real-time RT-PCR was used to quantify the expression of seven genes (dhcr 24, npc1l1, scap, dhcr7, leptin, soat1 and cyp51 genes) that were differentially expressed in RT2 PCR array. Real time RT-PCR results were in agreement with RT2 array gene expression data (Fig 8). More specifically, the expressions of dhcr24, dhcr7, npc1l1 and soat1 genes were significantly increased in HFD-fed animals; however, these genes were significantly suppressed in HFD + DSHT-treated animals. Notably, Scap gene expression was decreased in HFD-fed mice with respect to NOR, but significantly increased upon treatment with DSHT. Leptin gene expression was higher in HFD-fed mice compared to NOR. However, significant reduction in the expression of this gene was observed in HFD + DSHT-treated animals compared to HFD group. The expression of the cyp51 gene was higher in HFD-fed animals vs. NOR, which was further elevated upon DSHT treatment.
Fig 8. Quantification of gene expression using real-time RT-PCR.
To confirm the gene expression of RT array gene expressions, the gene expression of seven differentially expressed genes was evaluated using real-time RT-PCR. Relative gene expression was performed using 2-Δct method and the fold changes in gene expression were normalized with the GAPDH gene. Data were represented as mean ± SD (n = 3).
Discussion
The potential of natural products for treatment of obesity is under exploration, and this may be an excellent alternative strategy for development of future effective and safe anti-obesity drugs. We previously reported on various anti-obesity herbal medicine formulations including Flos Lonicera [22], Rhizoma Atractylodis Macrocephalae [20], Bofutsushosan [23], and Rehmannia glutinosa [24]. In this study, we evaluated the anti-obesity property of DSHT using HFD-induced obese mice as a model.
In animals, feeding a HFD induces hypertrophy and hyperplasty of adipocytes, leading to total body weight gain which ultimately results in obesity [25]. In this study, mice fed a HFD gained significantly higher weight than those fed a normal diet indicating that the diet-induced obesity was successfully achieved. However, the mice fed on HFD along with oral supplementation of DSHT did not demonstrate significant weight gain, indicating the anti-obesity impact of DSHT. A similar kind of impact on HFD-fed mice was also shown by ORL, in keeping with recent reports revealing a potent inhibitory effect of orlistat on body weight gain in HFD-induced obese rats and mice [26, 27]. Furthermore, there was significant reduction in liver weight, intestinal fat and total fat in HFD + DSHT-treated mice compared to HFD-fed animals, suggesting that DSHT effectively attenuated proliferation or differentiation of adipose tissues. The total fat mass directly correlated with the body weight and body weight gain; thus, it can be concluded that DSHT suppressed body weight gain by repressing the expansion of adipose tissue mass [28]. In contrast, in HFD group, although ORL significantly decreased the intestinal fat, it failed to produce any significant change in the liver weight and total fat.
It has been found that the serum leptin concentration is directly proportional to the amount of adipose tissue mass [29]. In this study, the leptin protein encoding gene, leptin, was insignificantly, but markedly up-regulated in HFD-fed animals, which however, was down-regulated in both HFD+DSHT and HFD+ORL groups. Leptin is an important regulator of the mass of adipose tissue and body weight, and overexpression of leptin gene has been reported in subcutaneous adipose tissue of massively obese persons [30]. Our findings are also in keeping with a recent study demonstrating depletion in serum leptin concentration in HFD-fed rats in response to the treatment with orlistat [27]. Another obesity-related gene, adiponectin, has been associated with obesity and insulin resistance [31]. In humans, plasma adiponectin concentrations decrease with increasing obesity, and reduced adiponectin concentrations correlate with insulin resistance and hyperinsulinemia [32]. Here expression of the adiponectin gene was lower in HFD group vs. NOR group, but its expression was significantly increased in HFD+DSHT-treated animals, but not in HFD+ORL group.
A previous report has suggested that adipocyte dysfunction-linked obesity imparts insulin resistance and diabetes [33]. In this study, mice fed a HFD showed marked increase in serum insulin and glucose levels and AUC of the glucose response in OGTT. Likewise, elevated serum levels of TC, TG, and GPT and a pronounced decrease in serum HDL level were observed in HFD group compared to NOR group. However, treatment with DSHT, but not ORL, significantly reduced the serum glucose levels and AUC of the glucose response in OGTT in HFD-fed animals, producing a hypoglycemic effect. Importantly, the serum insulin level was also significantly decreased in HFD group in response to treatment with DSHT, but not ORL, endeavoring a protective effect of the proposed herbal formulation on beta cell deterioration [34]. Thus our study suggests the beneficial impact of DSHT on glucose homeostasis and metabolism in HFD-induced obesity. The reasons behind the inability of orlistat in exerting such effects on HFD-fed mice are yet to be clarified. Nevertheless, our findings are in alignment with a previous study revealing that single dose of 120-mg orlistat did not cause any change in postprandial serum glucose, insulin or glucagon-like peptide-1 levels in nondiabetic obese patients [35]. While the levels of aberrant biochemical parameters including TC, TG, HDL, and GPT in HFD-fed mice were either ameliorated or remained unchanged/or normalcy was restored upon treatment with either DSHT or ORL. Based on these, it is conceivable that DSHT and ORL may improve the homeostasis of lipid parameters in HFD-induced obese state. Recent studies have demonstrated that orlistat treatment results in marked improvements in serum and hepatic lipid profiles including TG, TC, low-density lipoprotein cholesterol, and HDL cholesterol in HFD-induced obese rats or mice [26, 27]. Collectively, our findings indicate that DSHT combats body weight gain, reduces total body fat, regulates the expressions of leptin and adiponectin genes in adipose tissue, and exerts an anti-diabetic effect by maintaining glucose homeostasis. The marked improvement in the histopathological characteristics of hepatic tissue in HFD-fed mice by DSHT treatment further supports the anti-obesity impact of DSHT.
Substantial evidence indicate that the gut microbiota play significant roles in diabetes and obesity by influencing energy extraction, inflammation, and neuroendocrine secretions [7, 36–43]; in agreement with our previous findings and other reports revealing that a modulation in the gut bacteria may be linked to obesity [20, 22, 23, 24, 44]. The human gut microbiota encompass more than 1000 phylotypes [43], and it has been estimated that 99% of them are represented by five phyla; Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, and Proteobacteria. Among them, Bacteroidetes and Firmicutes account for more than 90% of bacterial population [43]. Growing evidence from clinical studies have revealed that obese subjects with insulin resistance are characterized by an altered composition of gut microbiota, particularly an elevated Firmicutes/Bacteroidetes ratio compared with healthy people [45, 46]. This is further supported by the fact that transplantation of the obese gut microbiota in animals significantly affected the energy harvest of hosts [46]. More specifically, it has been proposed that altered microbiota in obesity modulates intestinal permeability and facilitates metabolic endotoxin secretion that cause chronic low-level inflammation, the hallmarks of insulin resistance and onset of type 2 diabetes [47, 48]. In this study, the PCR-DGGE fingerprinting analysis indicated an alteration in gut microbiota in HFD-fed mice with respect to NOR. While, the banding patterns showed similarities among HFD + DSHT, HFD + ORL, and NOR groups. Therefore, it is conceivable that DSHT treatment probably prevented the aberration of microbial community and restored normalcy in bacterial population in HFD-fed animals. These results were further confirmed by PCA analysis of DGGE fingerprinting. In this analysis, HDF + DSHT-treated animals were clustered close to HFD + ORL and NOR groups, indicating a strong resemblance in the gut microbial communities among these three groups.
To further evaluate the changes in abundance of gut microbiota in HFD-fed mice in response to DSHT treatment, qRT-PCR was performed for quantification of the intestinal flora in stool samples. Our results showed changes in the relative abundances of gut microbial population in response to feeding HFD, mostly in a significant manner. An alteration in gut microbiota at the level of phylum or genus in obesity has been reported in various studies [49, 50]. Based on these evidence, it is conceivable that an imbalance in the abundances of gut bacteria plays a key role in the onset and development of obesity which is likely to be mediated through a number of factors, including boosting of energy harvest from diet, induction of systemic inflammation, and acceleration of fat deposition [51, 52]. Our findings are also in agreement with previous studies where it have been noticed that an increase in the level of Firmicutes and a decrease in the population of Bacteroidetes, an imbalance in Bacteroidetes/Firmicutes ratio, and destabilization of Akkermansia population are linked to obesity [48, 53–55]. Similarly, reduced numbers of Bifidobacterium, and Lactobacillus have been associated with obesity [56, 57].
Accumulating evidence indicate that normalization of the gut microbial population can ameliorate obesity and other associated metabolic alterations and immune dysfunction in animal models [58, 59]. In agreement with this, we found that anti-obesity effects of both DSHT and ORL on HFD-fed mice were associated with restoration of vital gut microbial members as mentioned above. This is also further supported by our findings on correlations between relative abundances of the gut microbial communities and vital metabolic parameters related to obesity. Both Bacteroidetes and Bacteroidetes/Firmicutes ratio showed a definite negative correlation with TC. Lactobacillus and Roseburia exhibited significant negative correlations with the body and liver weights. Lactobacillus also demonstrated a significant negative correlation with the total body fat and an insignificant, but obvious negative correlation with serum TC level. While, Akkermansia showed an insignificant, but a prominent negative correlation with serum TG level. Ruminococcus revealed insignificant, but clear negative correlations with body weight, liver weight, and serum TC level. Bacteroides on the other hand exhibited a significant negative correlation with liver weight and insignificant, but definite negative correlations with body weight and serum TC level. These are in agreement with a recent study which has supported the significance of therapies altering the gut microbiome to regulate body mass, TG and HDL [60]. Additionally, a number of clinical studies have indicated an association between the adiponectin level and obesity-related metabolic dysfunction [61]. More specifically, dysregulation of adipokines has been demonstrated to be associated with obesity, type 2 diabetes, and hypertension [62] These all are in keeping with our findings where significant or marked positive correlations were found between the serum HDL or adiponectin levels or both of these parameters with the relative abundances of Bacteroides, Prevotella, Akkermansia, Lactobacillus, Roseburia, and Ruminococcus. Taking all in consideration, our findings suggest that DSHT or ORL ameliorates HFD-induced obesity partly through restoring the balance in the gut microbial community.
We hypothesize that probable prebiotic effects of DSHT may contribute to the regulation of gut microbial population. It has been found that prebiotics are non-digestible complex carbohydrates that are fermented in the colon, producing energy and short chain fatty acids, and selectively promote the growth of beneficial gut bacteria [63]. Several plants and plant products such as vegetables, root and tuber crops as well as some fruit crops are the well-known sources of prebiotic carbohydrates. So far, fructooligosaccharides (FOS), inulin, and galactooligosaccharides (GOS) from plants are identified as the rich sources of prebiotics. Additionally, the raffinose family of oligosaccharides and resistant starch (the type that is not allowed for absorption in the gastrointestinal tract) has also been found as prebiotic carbohydrates as these molecules are not absorbed in the intestine and facilitate the growth of beneficial gut bacteria [64, 65]. Moreover, some plant cell wall polysaccharides, including xylans and pectins, have also been identified as the chief sources for diverse polysaccharides to generate new prebiotics [66].
Accumulating evidence indicate that multiple indigestible dietary carbohydrates acting as prebiotics can selectively induce the growth of a subset of beneficial gut bacteria and subsequently maintain the homeostasis of gut microbial community as well as the host health [46, 67]. For instance, phylum Bacteroidetes always possess several PULs (polysaccharide utilization loci)-encoded products, which are collectively termed as Sus (starch utilization system)-like systems. These systems encompassing several to decades of enzymes are involved in the degradation of different polysaccharides by targeting specific glycosidic linkages or chemical substituents in the polysaccharides [68]. It has been found that unlike Bacteroidetes, Firmicutes and Actinobacteria express very few carbohydrate-degrading enzymes but enriched with polysaccharide-specific ABC (ATP-binding cassette) transporters which play an important role in the transportation of degraded carbohydrates [69]. This might largely account for the variation of phylum Firmicutes and Actinobacteria in response to the treatment with ginseng polysaccharides as revealed in a previous study [70].
Since DSHT is a multi-herbal medicine, the presence of a variety of prebiotic carbohydrates in this formulation is not an unexpected phenomenon. However, future detailed studies are needed to trace the prebiotics present in DSHT and to elucidate their mode of action in the regulation of gut microbial population in order to validate our hypothesis.
Adipocyte proliferation and differentiation are regulated by a complex network of genes. Accordingly, to identity the potential target genes of DSHT, the expression levels of vital genes regulating lipid and cholesterol metabolism were evaluated in animals treated with HFD either alone or in combination with DSHT or ORL. The results showed that 39 (46%) genes were differentially expressed in HFD-fed mice compared to NOR group. However, no significant changes in gene expression vs. NOR (or restoration of normal gene expression) was shown by in 36 (92%) genes in HFD + DSHT treated animals, indicating that DSHT modulates the vital genes involved in lipid and cholesterol metabolism.
Our study showed the up-regulation of vital lipid and cholesterol metabolism genes- apoa4, ncp1l1, srebf1, cyp11a1, cyp46a and cyp51 in HFD-fed mice. The expressions of these genes were either decreased or unchanged in HFD + DSHT-treated animals vs. NOR group. More specifically, the intestinal cholesterol absorption protein encoding gene, ncp1l1 was up-regulated by >10 folds in HFD-fed mice, however, its expression was decreased to 1-fold in HFD + DSHT-treated animals vs. NOR group. It has been found that ncp1l1 protein plays an essential role in intestinal cholesterol absorption [71]. Growing evidence strongly suggests that ncp1l1 inhibition have beneficial impact on the components of metabolic syndrome, such as obesity, insulin resistance, fatty liver, in addition to atherosclerosis [72]. While, the srebf1 gene encodes the sterol regulatory element binding factor 1 that controls the expression of fatty acid, phospholipid, and triglyceride biosynthetic genes, and the increased expression of srebf1 was found to be related to obesity [73].
In contrast, the expressions of vldlr, stab1, and srebp2 genes were suppressed in HFD-fed animals but restored to normal when treated with DSHT. The stab1 gene encodes stabilin-1 protein which is involved in the complex physiological clearance processes, up-taking and degradation of the unwanted self-molecules and routing of endogenous and exogenous endocytosis ligands to the secretory pathways [74]. srebp (sterol-regulatory element-binding protein)2 is another important gene down-regulated in HFD-fed mice, but restored to 1.9 fold in HFD + DSHT-treated animals. It has been found that srebp is a key regulator of cholesterol homoeostasis, and the reduced expression of this gene may lead to decreased transcriptional activation of the LDL receptor-gene and elevated plasma TC level [75]. Collectively, our gene expression results signify that DSHT treatment regulates the expression of genes involved in NAFLD, fat digestion and absorption, insulin signaling pathway, PPAR signaling pathway, AMPK signaling pathway, metabolic pathway, steroid biosynthesis, terpenoid back-bone biosynthesis, low-protein partical and low-density lipoprotein binding and receptor activity. Probably, the anti-obesity effect of DSHT may be, at least in part, due to the regulation of vital genes involved in cholesterol and lipid metabolism. Notably, we also found that the food efficiency ratio, which was higher in HFD group vs. NOR group, decreased significantly in response to the treatment with either DSHT. Further studies are needed to elucidate whether the altered expression of the above mentioned genes are linked to this effect of DSHT.
Conclusion
The results of this study demonstrate that DSHT is an effective anti-obesity herbal formulation against HFD-induced obesity. DSHT attenuates obesity in mice through the alteration of gene expressions and modulation of intestinal microbiota. In HDF-fed animals, this formulation reduced the fat deposition, TC, TG, and fasting glucose levels, AUC of the blood glucose response in OGTT, and elevated the HDL level. DSHT also regulates the expression of adipose- related genes, which in turn probably modulates the distribution of the intestinal flora and restores the relative abundances of vital bacteria including Lactobacillus, Akkermansia, Bifidobacterium as well as Bacteroidetes/Firmicutes ratio, and prevents the imbalance of gut microbiota in obese mice. Furthermore, it also regulates a number of vital genes involved in cholesterol and lipid metabolism.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This study was supported by a grant from the Traditional Korean Medicine R&D Project, Ministry for Health & Welfare & Family Affairs, Republic of Korea (HI13C0530).
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by a grant from the Traditional Korean Medicine R&D Project, Ministry for Health & Welfare & Family Affairs, Republic of Korea (HI13C0530). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766–81. Epub 2014 May 29 10.1016/S0140-6736(14)60460-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World Health Organization, 2015. Obesity and overweight http://www.who.int/mediacentre/factsheets/fs311/en/. 2015. 26098405 PMCID: PMC4625533
- 3.Yang L, Colditz GA. Prevalence of overweight and obesity in the united states, 2007–2012. JAMA Intern Med. 2015;175(8):1412–3. 10.1001/jamainternmed.2015.2405 . PMCID: PMC4625533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guh D, Zhang W, Bansback N, Amarsi Z, Birmingham C, Anis A. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health. 2009; 9(1): 1–20. 10.1186/1471-2458-9-88 PMCID: PMC2667420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nature cell biol. 2015; 17(4): 376–85. Epub 2015 Mar 2 10.1038/ncb3122 PMCID:PMC4380653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011; 12(11): 722–34. 10.1038/nrm3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre M, Venema K. The art of targeting gut microbiota for tackling human obesity. Genes & Nutr. 2015; 10(4): 20 Epub 2015 May 20. 10.1007/s12263-015-0472-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaoutari AE, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Micro. 2013; 11(7): 497–504. Epub 2013 Jun 10 10.1038/nrmicro3050 [DOI] [PubMed] [Google Scholar]
- 9.Yun JW.Possible anti-obesity therapeutics from nature–A review. Phytochemistry. 2010; 71(14): 1625–1641. 10.1016/j.phytochem.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 10.Guerciolini R. Mode of action of orlistat. Int J Obes Relat Metab Disord.1997; 21 (3): S12–23., [PubMed] [Google Scholar]
- 11.Bray G. A. A concise review on the therapeutics of obesity. Nutrition, 2000; 16(10): 953–960) 10.1016/S0899-9007(00)00424-X [DOI] [PubMed] [Google Scholar]
- 12.Butsch WS. Obesity medications: what does the future look like? Current Opinion in Endocrinology, Diabetes and Obe. 2015; 22(5): 360–6. 10.1097/med.0000000000000192 . [DOI] [PubMed] [Google Scholar]
- 13.Park SD, Yun BG. Effects of Daesiho-tang and its component groups on diabetes, free radical and antioxidative defense system in alloxan induced diabetic rats. Korean J Orient Med Prescr. 2001; 9(1): 289–317. [Google Scholar]
- 14.Kim CH, Kweon YM, Lee YT, Park SD. The preventive effect of Daesiho-tang on liver damage induced by acetaminophen in the rats. Korean J Orient Med Prescr.2004; 12(2): 139–154. [Google Scholar]
- 15.Yeo UH, J HJ, Kim HH. Effects of Daesiho-tang extract on Hypertension and Arterial Contraction. Korean J. Oriental Physiology & Pathology. 200519(6): 1573–1579, [Google Scholar]
- 16.Kim JH, Chung SH, Shin GC, Moon IS, Lee WC. The effect of daisiho-tang extract on hypoxic e18 cortical neuroblast. J Korean Ori Med. 2001; 22(1): 63–77. [Google Scholar]
- 17.Rha YG, Choi SH, Ahn KS, Moon JJ. Preventive and therapeutic effect of Daesiho-tang and its components on gallstone formation of mouse. J Orint Med Pathol. 1991; 6: 1–25. [Google Scholar]
- 18.The National Academies Press W, D.C., USA, Guide for the care and use of laboratory animals. In: The national academies press; W, D.C., USA, editor. 1996. [Google Scholar]
- 19.Su ML, He Y, Li QS and Zhu BH. Efficacy of acetylshikonin in preventing obesity and hepatic steatosis in db/db Mice. Molecules. 2016; 21(8): 976 10.3390/molecules/21080976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JH, Bose S, Kim HG, Han KS, Kim H. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci Rep. 2015; 5: 8391 10.1038/srep08391 . PMCID: PMC4329570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–D52. Epub 2014 Oct 28 10.1093/nar/gku1003 PMCID:PMC4383874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JH, Bose S, Kim GC, Hong SU, Kim JH, Kim JE, et al. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS One. 2014; 9(1): e86117 10.1371/journal.pone.0086117 . PMCID: PMC3901675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Bose S, Seo JG, Chung WS, Lim CY, Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: A randomized double-blind controlled clinical trial. Clin Nut. 2014; 33(6): 973–81. Epub 2013 Dec 3 10.1016/j.clnu.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Han K, Bose S, Kim YM, Chin YW, Kim BS, Wang JH, et al. Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food & Funct. 2015; 6(8): 2684–92. Epub 2015 Jul 3. 10.1039/C5FO00232J . [DOI] [PubMed] [Google Scholar]
- 25.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009; 5(3): e1000324 10.1371/journal.pcbi.1000324 PMCID: PMC2653640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju JE, Jegal J, Ahn J, Kim J, and Yang MH. Anti-obesity effect of dioscorea oppositifolia extract in high-fat diet induced obese mice and its chemical characterization. Biol. Pharm. Bull. 2016; 39(3): 409–414. 10.1248/bpb.b15-00849 [DOI] [PubMed] [Google Scholar]
- 27.Karimi G, Sabran MR, Jamaluddin R, Parvaneh K, Mohtarrudin N, Ahmad Z, et al. The antiobesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr Res. 2015; 22(59): 29273 , PMCID:PMC4689799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pichiah PBT, Moon HJ, Park JE, Moon YJ, Cha YS. Ethanolic extract of seabuckthorn (Hippophae rhamnoides L) prevents high-fat diet–induced obesity in mice through down-regulation of adipogenic and lipogenic gene expression. Nutr Res. 2012; 32(11): 856–64. 10.1016/j.nutres.2012.09.015 . [DOI] [PubMed] [Google Scholar]
- 29.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. [DOI] [PubMed] [Google Scholar]
- 30.Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995; 1(9): 950–3. [DOI] [PubMed] [Google Scholar]
- 31.Bulló M, Salas-Salvadó J, García-Lorda P. Adiponectin expression and adipose tissue lipolytic activity in lean and obese women. Obes Surg. 2005; 15(3): 382–6. 10.1381/0960892053576776 . [DOI] [PubMed] [Google Scholar]
- 32.Greenberg AS, and Obin MS. "Obesity and the role of adipose tissue in inflammation and metabolism." Am j clin nutr. 2006; 83(2): 461S–465S. . [DOI] [PubMed] [Google Scholar]
- 33.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008; 9(5): 367–77. 10.1038/nrm2391 PMCID:PMC2886982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Jiang J, Yu P, Zeng X, Larrick J, Wang Y. Hypoglycemic and beta cell protective effects of andrographolide analogue for diabetes treatment. J of Transl Med. 2009; 7(1): 62 10.1186/1479-5876-7-62 PMCID:PMC3224956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin M, Tanaci N, Yucel M, Tutuncu NB, Guvener N. The effect of single-dose orlistat on postprandial serum glucose, insulin and glucagon-like peptide-1 levels in nondiabetic obese patients. Clin Endocrinol (Oxf) 2007; 67(3), 346–50. Epub 2007 May 24. 10.1111/j.1365-2265.2007.02888.x [DOI] [PubMed] [Google Scholar]
- 36.John K. Baise D, Frank D N, and Mathur R. Impact of the gut microbiota on the development of obesity: current concepts. Am J Gastroenterol Suppl. 2012; 1, 22–27, 10.1038/ajgsup.2012.5 [DOI] [Google Scholar]
- 37.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010; 33(10): 2277–84. 10.2337/dc10-0556 , PMCID:PMC2945175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross PR, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92(1087): 286–300. 10.1136/postgradmedj-2015-133285 . [DOI] [PubMed] [Google Scholar]
- 39.Boulangé C L, Neves AL, Chilloux J, Nicholson JK, and Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Medicine. 2016; 8(1): 42 10.1186/s13073-016-0303-2 , PMCID:PMC4839080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol. 2014; 29(5): 190 10.3389/fmicb.2014.00190. eCollection 2014. , PMCID: PMC4010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philippe G. Gut microbiota and obesity. Cel Mol Life Sci 2016, 73 (1): 147–162, 10.1007/s00018-015-2061-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox AJ, West NP, and Cripps AW. Obesity, inflammation, and the gut microbiota. The Lancet. 2015; 3(3): 207–215. 10.1016/S2213-8587(14)70134-2 [DOI] [PubMed] [Google Scholar]
- 43.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects of Med. 2013; 34(1): 39–58. Epub 2012 Nov 16 10.1016/j.mam.2012.11.001 . [DOI] [PubMed] [Google Scholar]
- 44.Cani PD. Gut microbiota and obesity: lessons from the microbiome. Brief Funct Genomics. 2013; 12(4): 381–387, 10.1093/bfgp/elt014 . [DOI] [PubMed] [Google Scholar]
- 45.Tilg H, and Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6): 2126–2132, 10.1172/JCI58109. Epub 2011 Jun 1, , PMCID: PMC3104783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tremaroli V, and Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012; 489(74259): 242–249, 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 47.Diamant M, Blaak EE, Vos de WM. Do nutrient–gut–microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes?.Obesity Reviews. 2011; 12(4): 272–281, 10.1111/j.1467-789X.2010.00797.x [DOI] [PubMed] [Google Scholar]
- 48.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Prac & Res Clin Gastroenterology. 2013; 27(1): 73–83. 10.1016/j.bpg.2013.03.007. . [DOI] [PubMed] [Google Scholar]
- 49.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009; 457(7228): 480–4. Epub 2008 Nov 30 10.1038/nature07540 PMCID: PMC2677729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444(7122): 1027–3. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 51.Bajzer M, Seeley RJ. Physiology: Obesity and gut flora. Nature. 2006; 444(7122): 1009–10. 10.1038/4441009a [DOI] [PubMed] [Google Scholar]
- 52.Tsai F, Coyle WJ. The microbiome and obesity: Is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009; 11(4): 307–13. 10.1007/s11894-009-0045-z [DOI] [PubMed] [Google Scholar]
- 53.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005; 102(31): 11070–5. Epub 2005 Jul 20. PMCID: PMC1176910. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal Akkermansia muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS One. 2013; 8(9): e74963 10.1371/journal.pone.0074963 . PMCID: PMC3769299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006; 444(7122): 1022–3. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 56.Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015; 10(5): e0126931 10.1371/journal.pone.0126931 PMCID: PMC4436290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanz Y, Rastmanesh R, Agostonic C. Understanding the role of gut microbes and probiotics in obesity: How far are we? Pharmacol Res. 2013; 69(1): 144–55. Epub 2012 Nov 9. 10.1016/j.phrs.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 58.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opinion in Pharmacol. 2009; 9(6): 737–43. Epub 2009 Jul 2. 10.1016/j.coph.2009.06.016 . [DOI] [PubMed] [Google Scholar]
- 59.Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity. 2013; 21(11): 2310–21. Epub 2013 May 29 10.1002/oby.20330 [DOI] [PubMed] [Google Scholar]
- 60.Fu J, Bonder MJ, Cenit MC, Tigchelaar E, Maatman A, Dekens JAM, et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circul Res. 2015. 9; 117(9): 817–24. Epub 2015 Sep 10 10.1161/CIRCRESAHA.115.306807 . PMCID: PMC4596485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abella V, Scotece M, Conde J, López V, Lazzaro V, Pino J, et al. Adipokines, Metabolic Syndrome and Rheumatic Diseases. J of Immunol Res. 2014; 2014:14 Epub 2014 Feb 26. 10.1155/2014/343746 PMCID: PMC3987880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann NY Acad of Sci. 2010; 1212: E1–E19. 10.1111/j.1749-6632.2010.05875.x PMCID: PMC3075414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dwivedi S, Sangam K; Sahrawat, Puppala N, Ortiz R. Plant prebiotics and human health: Biotechnology to breed prebiotic-rich nutritious food crops Electronic Journal of Biotechnology, 2014, 17(5)7: 238–245. [Google Scholar]
- 64.Fuentes ZE, Sánchez ZE, Sendra E, Sayas E, Navarro C, Fernández L J, et al. Resistant starch as prebiotic: A review. Starch-Starke. 2011; 63: 406–15. 10.1002/star.201000099 [DOI] [Google Scholar]
- 65.Van den E W. Multifunctional fructans and raffinose family oligosaccharides. Front Plant Sci. 2013;4:247. 10.3389/fpls.2013.00247: ,PMCID:PMC3713406 10.3389/fpls.2013.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo HD, Kim D, Paek SH. Plant cell wall polysaccharides as potential resources for the development of novel prebiotics. Biomol her (Seoul) 2012; 20(4): 371–9 10.4062/biomolther.2012.20.4.371 , PMCID: PMC3762269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koropatkin NM., Cameron EA. & Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012; 10: 323–335. 10.1038/nrmicro2746 , PMCID:PMC4005082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martens E.C. Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PlosBiol. 2011; 9(12): e1001221 10.1371/journal.pbio.1001221 , PMCID: PMC3243724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahowalda MA., Reya FE., Seedorfa H, Turnbaugha PJ., Fultonb RobS., Wollamb A, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci USA. 2009. 106(14): 5859–5864, 10.1073/pnas.0901529106 PMCID: PMC2660063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou SS, Xu J, Zhu H, Wu J, Xu JD, Yan R, et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Re. 2016; 6:22474 10.1038/srep22474 PMCID: PMC4774164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis HR, Zhu Lj, Hoos LM, Tetzloff G, Maguire M, Liu J, et al. Niemann-Pick C1 Like 1 (NPC1L1) Is the Intestinal Phytosterol and Cholesterol Transporter and a Key Modulator of Whole-body Cholesterol Homeostasis. J Biol Chem. 2004;279(32):33586–92. Epub 2004 Jun1. 10.1074/jbc.M405817200 [DOI] [PubMed] [Google Scholar]
- 72.Betters JL, Yu L. NPC1L1 and Cholesterol Transport. FEBS letters. 2010; 584(13): 2740–7. Epub 2010 Mar 19. 10.1016/j.febslet.2010.03.030 PMCID: PMC2909875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metabol. 2012; 15(2): 240–6. 10.1016/j.cmet.2011.12.017 , PMCID: PMC3662050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kzhyshkowska J, Gratchev A, Goerdt S. Stabilin-1, a homeostatic scavenger receptor with multiple functions. J Cell Mol Med. 2006; 10(3): 635–49. 10.1111/j.1582-4934.2006.tb00425.x PMCID: PMC3933147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miserez AR, Muller PY, Barella L, Barella S, Staehelin HB, Leitersdorf E, et al. Sterol regulatory element-binding protein (SREBP)-2 contributes to polygenic hypercholesterolaemia. Atherosclerosis. 2002; 164 (1): 15–26. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper.