Abstract
Purpose of Review
Hypothyroidism is highly prevalent in chronic kidney disease (CKD) patients, including those receiving dialysis. This review examines potential mechanistic links between thyroid and kidney disease; current evidence for hypothyroidism as a risk factor for de novo CKD and CKD progression; and studies of thyroid functional disorders, cardiovascular disease, and death in the CKD population.
Recent Findings
Epidemiologic data have demonstrated an incrementally higher prevalence of hypothyroidism with increasing severity of kidney dysfunction. Various thyroid functional test abnormalities are also commonly observed in CKD, due to alterations in thyroid hormone synthesis, metabolism, and regulation. While the mechanistic link between thyroid and kidney disease remains unclear, observational studies suggest hypothyroidism is associated with abnormal kidney structure and function. Previously thought to be a physiologic adaptation, recent studies show that hypothyroidism is associated with higher risk of cardiovascular disease and death in CKD.
Summary
A growing body of evidence suggests that hypothyroidism is a risk factor for incident CKD, CKD progression, and higher death risk in kidney disease patients. Rigorous studies are needed to determine impact of thyroid hormone replacement upon kidney disease progression, cardiovascular disease, and mortality, which may shed light into the causal implications of hypothyroidism in CKD.
Keywords: Thyroid function, thyrotropin, hypothyroidism, hyperthyroidism, chronic kidney disease, dialysis
Introduction
Thyroid functional disorders are commonly observed in chronic kidney disease (CKD) patients.[1] Primary hypothyroidism, which is typically identified by biochemical tests including an elevated serum thyrotropin (TSH) level in conjunction with a low or normal thyroxine (T4) level (defined as overt and subclinical hypothyroidism, respectively),[2] is disproportionately more prevalent in patients with advanced kidney dysfunction compared to those with normal function.[3] In addition, various thyroid functional test abnormalities are frequently seen in CKD patients, resulting from alterations in thyroid hormone synthesis, metabolism, and regulation.[1, 4] While early studies hypothesized that thyroid hormone deficiency may be a physiologic adaptation in kidney disease patients,[5] contemporary data have demonstrated that hypothyroidism is associated with higher risk of cardiovascular disease and death in this population.[1, 6–9] In this review, we will (i) examine potential mechanistic links between thyroid and kidney disease; (ii) describe common patterns of thyroid functional test alterations in CKD; (iii) summarize existing evidence of thyroid hormone deficiency as a risk factor for cardiovascular disease and death in CKD, including underlying pathophysiologic mechanisms; and (iv) discuss the clinical management of hypothyroidism in CKD patients and future areas of research.
Hypothyroidism: A Common Yet Under-Recognized Endocrine Disorder in Kidney Disease
While a greater emphasis has been placed upon other endocrine disorders in CKD (e.g., secondary hyperparathyroidism, diabetes), large observational studies show that hypothyroidism is highly prevalent in kidney disease patients. For example, among 14,623 participants in the Third National Health and Nutritional Examination Survey (NHANES III), there was an incrementally higher prevalence of hypothyroidism (defined as TSH >4.5 mIU/L or receipt of exogenous thyroid hormone supplementation) with increasing severity of kidney dysfunction: 5, 11, 20, 23, and 23% with estimated glomerular filtration rates (eGFRs) of ≥90, 60–89, 45–59, 30–44, and <30 ml/min/1.73m2, respectively.[3] Even after accounting for differences in age, sex, and race/ethnicity, participants with eGFRs <30 ml/min/1.73m2 had a 2-fold higher risk of hypothyroidism compared to those with eGFRs >90 ml/min/1.73m2. In a more recent study of 461,607 US veterans with Stages 3 to 5 CKD who underwent serum TSH testing from 2004–6 (84% of the cohort), 23% had hypothyroidism (defined as TSH >5.0 mIU/L or receipt of exogenous thyroid hormone replacement).[10] Across these studies, a large proportion of cases have been due to subclinical hypothyroidism. In the aforementioned NHANES III study, 56% of hypothyroid cases were due to subclinical disease.[3] In a study of 3089 ambulatory adults in Italy, it was also found that there was an increasingly higher prevalence of subclinical hypothyroidism with lower levels of kidney function: 7 vs. 18% with eGFRs ≥90 vs. <60 ml/min/1.73m2, respectively.[11]
A high prevalence of hypothyroidism has also been observed in dialysis patients, albeit in comparatively smaller-sized cohorts. In US and Asian hemodialysis and peritoneal dialysis cohorts, the prevalence of hypothyroidism has ranged from 13 to 25%.[9, 12–14] Despite these data, hypothyroidism remains under-recognized in many advanced CKD patients, likely due to symptom overlap with uremia (e.g., fatigue, cold intolerance, decreased cognition).[1, 3]
Mechanistic Links Between Thyroid and Kidney Disease
Thyroid Functional Disease as a Risk Factor for Kidney Disease
While the mechanistic link between thyroid and kidney disease remains unclear, animal studies have demonstrated that hypothyroidism adversely affects kidney size and structure in both development and adulthood (Figure 1).[15] In neonatal rats, hypothyroidism has resulted in reduced kidney-to-body weight ratio, truncated tubular mass, and various glomerular basement membrane (GBM) changes (e.g., reduced GBM volume and area, GBM thickening, mesangial matrix expansion, and increased glomerular capillary permeability).[15–24] It has also been suggested that hypothyroidism leads to kidney dysfunction, vis-à-vis several potential mechanisms, including (1) decreased cardiac output, (2) altered intra-renal hemodynamics (i.e., intra-renal vasoconstriction resulting from decreased vasodilator synthesis and activity), (3) reduced renin-angiotensin-aldosterone production and activity, and (4) increased tubulo-glomerular feedback due to alterations in chloride channel and expression.[15, 23, 25–28] Indeed, in animal studies, induction of hypothyroidism by thyroidectomy has resulted in reduced single nephron glomerular filtration rate, renal plasma flow, and glomerular transcapillary pressure.[29, 30] Multiple case series have also shown that severe hypothyroidism results in creatinine elevations, reduced plasma flow, and decreased GFR as ascertained by indirect estimating equations, and gold standard isotopic scans; in these reports, creatinine elevations and reductions in GFR were reversed with exogenous thyroid hormone replacement.[31–33]
Figure 1. Proposed mechanistic links between thyroid and kidney disease.
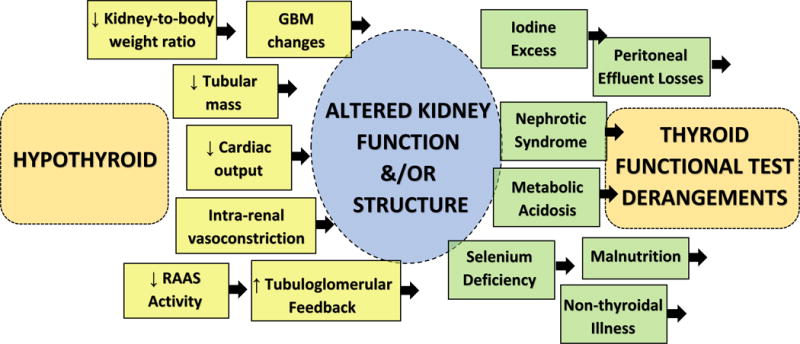
Abbreviations: GBM, glomerular basement membrane; RAAS, renin angiotensin aldosterone system.
Some, but not all, epidemiologic studies have corroborated an association between hypothyroidism and kidney dysfunction. In a cross-sectional analysis of 461,607 US veterans who underwent repeated measures of serum TSH and creatinine testing at identical time points, each 10 ml/min/1.73m2 decrement in eGFR was associated with an 18% higher risk of hypothyroidism, independent of socio-demographics and comorbidities.[10] In a prospective cohort study of 104,633 South Korean patients from the Kangbuk Samsung Health Study who had normal baseline kidney function and serum TSH levels and underwent annual to biennial TSH testing, those in the highest TSH quintile (2.85–5.00 mIU/L) had a 26% higher risk of incident CKD (defined as eGFR <60 ml/min/1.73m2 using the CKD Epidemiology Collaboration formula) compared to those in the lowest TSH quintile (0.25–1.18 mIU/L).[34] In analyses that examined TSH across a continuous spectrum using restricted cubic splines, higher TSH levels were associated with a progressively higher risk of CKD up to a TSH level of ~3.20 mIU/L above which risk plateaued, suggesting that even high-normal TSH levels may be a risk factor for kidney dysfunction. However, the thyroid dysfunction—CKD association has not been confirmed in all subpopulations. For example, in an analysis of 558 participants in the Leiden-85 study, a prospective follow-up study of 85-year old inhabitants of Leiden, the Netherlands, cross-sectional analyses showed that those with overt and subclinical hypothyroidism (defined as TSH >4.5mIU/L with free T4 levels in the low or normal range, respectively) had lower mean ± SE eGFR levels than participants with normal kidney function: 53.7 ± 2.0, 55.7 ± 2.1, and 59.5 ± 0.7 ml/min/1.73m2; in contrast, participants with hyperthyroidism had the highest eGFR levels: 61.5 ± 3.1 ml/min/1.73m2.[35] In addition, patients with higher TSH levels and lower free T4 levels had lower kidney function at baseline. However, in longitudinal analyses, there was no association between baseline thyroid functional status nor thyroid hormone concentrations with change in eGFR over a median follow up of five years.
Limited data also suggest that, among patients with “mild” subclinical hypothyroidism and CKD, thyroid hormone replacement may ameliorate kidney disease progression.[36, 37] Among 309 patients with subclinical hypothyroidism and Stages 2 to 4 CKD, compared to untreated patients (42%), those who were treated (58%) had a slower rate of eGFR decline (−2 vs. −6 ml/min/1.73m2/year, respectively) and were less likely to experience a 50% decline in eGFR or end-stage renal disease (adjusted HR [95% CI] 0.28 [0.12–0.68], respectively).[36] In light of the sparse modifiable factors for CKD progression, further rigorous studies examining whether thyroid-hormone replacement therapy modulates kidney function trajectory in hypothyroid CKD patients are needed.
Chronic Kidney Disease as a Risk Factor for Thyroid Dysfunction
Conversely, patients with CKD may be at higher risk for thyroid dysfunction via several suggested pathways (Figure 1). For example, iodine retention due to impaired kidney excretion has been hypothesized as a possible mechanism for hypo- and hyperthyroidism in CKD via the Wolff-Chaikoff effect and Jod-Basedow phenomenon, respectively.[38] Several case reports and series have reported iodine-associated hypothyroidism among adult and pediatric dialysis patients consuming high iodine diets (e.g., seaweed), exposure to povidine-iodine cleansing agents, and receipt of iodine-contrast media enhanced angiography.[39–42] While several recent observational studies have shown that iodinated contrast media is associated with higher risk of incident hypothyroidism and hyperthyroidism, a more potent association in those with vs. without kidney dysfunction has not been observed.[43–45] Patients with CKD may frequently have metabolic acidosis, which has been shown to result in thyroid functional test alterations (elevated TSH and low T4 and triiodothyronine [T3]) levels, partially improved with oral sodium citrate therapy.[46, 47] Selenium is an important trace mineral in the human diet that modulates the peripheral conversion of T4-to-T3 by controlling iodothyronine deiodinase activity.[48] However, in a double-blind randomized controlled trial of 64 hemodialysis patients with selenium deficiency who received oral selenium supplementation vs. placebo, there were no significant differences in TSH, T4, or T3 resin uptake levels after three months.[49] Lastly, given that the vast majority of circulating thyroid hormone is protein-bound, it has been suggested that heavy protein losses in nephrotic syndrome or via the dialysate in peritoneal dialysis patients may lead to total body thyroid hormone depletion.[50, 51]
Thyroid Functional Test Alterations in Chronic Kidney Disease
Under normal circumstances, thyroid hormone synthesis is under the regulation of the hypothalamic-pituitary-thyroid axis. Thyrotropin-releasing hormone (TRH) produced by the hypothalamus incites the release of TSH from the anterior pituitary, which in turn stimulates the synthesis and secretion of T4, and to a lesser degree T3, by the thyroid gland.[15] While T4 is generated in the thyroid gland, 80% of T3 is produced by the peripheral conversion of T4-to-T3 by type 1 and 2 5′-deiodinase enzymes. In a multi-loop feedback system, T4 and T3 exert negative feedback inhibition of TRH and TSH.
CKD patients may experience alterations in regulation of the hypothalamic-pituitary-thyroid axis. In addition, as the kidney is involved in the metabolism, degradation, and excretion of certain thyroid hormones and their metabolites, various thyroid functional test alterations may occur in CKD.[1, 4]
Thyrotropin
In the general population, serum TSH is typically used for screening, diagnosis, and treatment monitoring and titration in primary hypothyroidism.[2] In CKD, certain TSH alterations may be observed, such as impaired clearance, protracted half-life, blunted pulsatility, altered glycosylation leading to impaired bioactivity, and decreased response to TRH.[4, 52] Given its inverse logarithmic association with serum T3 and T4 levels (i.e., small changes in T3 and T4 induce exponential changes in TSH), it is regarded as the most sensitive and specific single biochemical measure of thyroid function. Additionally, serum TSH is a more robust thyroid function metric in non-thyroidal illness.[2] Whereas low T3 and T4 levels are typically observed with mild to moderate illness, TSH typically remains normal until the onset of severe, critical illness.[48, 53]
Triiodothyronine and Reverse Triiodothyronine
Limited data indicate that low T3 levels are the most commonly observed thyroid functional test alteration in CKD patients. For example, in a study of 2284 CKD patients who had normal TSH levels, there was an incrementally higher prevalence of low T3 with increasingly impaired kidney function, such that over three-fourths of patients with Stage 5 CKD had low levels: 8, 11, 21, 60, and 79% with eGFRs ≥90, 60–89, 30–59, 15–29, and <15 ml/min/1.73m2.[54] As the aforementioned peripheral deiodination of T4-to-T3 is reduced by malnutrition, inflammation, non-thyroidal illness, certain medications, and in the presence of high serum cortisol and free nonesterified fatty acid levels, it has been suggested that low T3 levels are a marker of illness in CKD patients.[10, 48, 53]
Reverse T3 is a metabolically inactive form of thyroid hormone that is produced from its precursor, T4, by the type 3 5′-deiodinase enzyme.[55, 56] This enzyme also degrades reverse T3 into inactive diiodothyronine. In non-thyroidal illness, reverse T3 levels are typically high due to heightened production and reduced decomposition.[4, 56] In hypothyroidism, reduced production of T4 typically results in low reverse T3 concentrations, although levels may be normal or high in mild hypothyroidism. While reverse T3 is oftentimes normal in kidney dysfunction, the clinical significance of these levels in the interpretation of thyroid functional tests in CKD remains undefined.[1]
Total and Free Thyroxine
Given that the vast majority of circulating T4 is bound to proteins, including thyroid-binding globulin, transthyretin, albumin, and lipoproteins, total T4 levels (capturing both free and protein-bound hormone) may be spuriously low in low-protein states.[57] While free T4 levels are used to ascertain the proportion of biologically active, non-protein bound hormone, routinely used free T4 assays (e.g., analog method) are dependent upon protein-hormone binding, and hence may not be accurate in abnormal protein states (e.g., hypoalbuminemia, pregnancy) or in the presence of certain medications (e.g., furosemide, heparin) or substances that impair protein-hormone binding (e.g., uremic toxins).[56, 57] To overcome this limitation, direct free T4 assays separate free and protein-bound T4 using equilibrium dialysis or ultrafiltration, and use liquid chromatography tandem mass spectrometry or radioimmunoassay to measure free T4.[57–59] While direct free T4 levels have shown stronger inverse correlations with the log of TSH compared with indirect FT4 in populations with normal and altered protein-hormone binding (e.g., pregnancy),[60–62] further studies are needed to determine their utility in the classification of thyroid function and prognostication of outcomes in CKD.
Thyroid Functional Disease and Outcomes in Chronic Kidney Disease
Cardiovascular Disease
In general population, hypothyroidism has been associated with various adverse cardiovascular outcomes (Table 1).[63] Given that end-stage renal disease patients have a 7 to 10-fold higher mortality risk (40% of deaths due to cardiovascular causes) compared to the general population,[64, 65] there has been increasing interest in hypothyroidism as an under-recognized cardiovascular risk factor in advanced CKD.[1] To date, most studies of thyroid function and cardiovascular endpoints in this population have focused upon low T3 as the thyroid functional test metric, which has been associated with endothelial dysfunction, atherosclerosis, vascular calcification, impaired systolic function, increased left ventricular mass, and abnormal ventricular conduction.[66–72] There have been comparatively fewer studies of thyroid function ascertained by TSH and cardiovascular outcomes in CKD. In a cross-sectional study of 51 ambulatory peritoneal dialysis patients, subclinical hypothyroidism (defined as TSH >5 mIU/L and normal free T4) was associated with impaired left ventricular function.[7] In a recent study of 97 end-stage renal disease patients selected to undergo living donor kidney transplantation, low TSH, free T3, and free T4 levels (interpreted as markers of non-thyroidal illness) were inversely associated with higher coronary artery calcification scores.[68]
Table 1.
Cardiovascular complications of hypothyroidism.
| CARDIOVASCULAR SYSTEM | SEQUELAE OF HYPOTHYROIDISM | PATHOGENESIS |
|---|---|---|
| Cardiac function |
|
|
| Endothelial function |
|
|
| Metabolic status |
|
|
|
|
|
| ||
| Vascular disease |
|
|
|
|
|
| Cardiac conduction |
|
|
Mortality
In the general population, studies of hypothyroidism (largely focused upon subclinical disease) and mortality have been mixed.[73] However, a meta-analysis of 55,287 patients across 11 cohort studies conducted by the Thyroid Studies Collaboration showed that patients with subclinical hypothyroidism and TSH levels >7 mIU/L had a higher risk of coronary heart disease death.[74] It has also been suggested that the hypothyroidism—mortality association may be dependent upon underlying cardiovascular risk. Indeed, in a study of 14,879 NHANES III participants, both hypothyroidism overall and subclinical hypothyroidism were associated with higher mortality in participants with heart failure, but not in those without heart failure.[13] These findings may bear analogy to advanced CKD patients given their high prevalence of structural heart disease.[64]
Early studies of thyroid function in CKD suggested that thyroid hormone deficiency is a physiologic adaptation and a means to conserve metabolism in end-stage renal disease patients who are hypercatabolic and prone to protein-energy wasting and dialytic protein and amino acid losses.[5] However, contemporary studies demonstrating an association between hypothyroidism and higher death risk in dialysis patients have challenged this hypothesis. In the first study to examine thyroid function defined by TSH and mortality, among 2715 incident+prevalent dialysis patients it was found that hypothyroidism ascertained at study entry (defined as TSH > assay reference upper limit of normal) was associated with a 35% higher all-cause mortality risk compared to euthyroidism.[8] Yet in a study of 1000 incident+prevalent diabetic hemodialysis patients from the Deutsche Diabetes Dialyse Studie (4D study), baseline subclinical hypothyroidism examined separately or in conjunction with overt hypothyroidism was not associated with sudden cardiac death, cardiovascular events, or all-cause death; notably, only 1.8% of the study cohort had subclinical hypothyroidism (defined as TSH 4.1–15.0 mIU/L with normal free T3 and T4 levels) or overt hypothyroidism (defined as TSH >10.0 IU/L and low free T3 and/or free T4 levels), which may have resulted in limited power to detect significant associations.[6] In a subsequent study of 8840 hemodialysis patients from a large national dialysis organization that examined baseline and repeated measures of TSH over time, it was found that baseline and time-dependent hypothyroidism (defined as TSH >5.0 mIU/L) were associated with a 47% and 62% higher all-cause death risk compared to euthyroidism, respectively.[9] Upon examination of finer gradations of TSH, it was found that higher levels were incrementally associated with higher death risk, such that even high-normal TSH ranges (>3.0–5.0 mIU/L) predicted heightened mortality risk. To explore the hypothesis that hypothyroidism is a physiologic adaptation in advanced CKD patients prone to protein-energy wasting, thyroid function—mortality associations were examined across strata of body mass index. Notably, time-dependent analyses showed stronger associations across higher body mass index categories, while baseline analyses showed that the hypothyroidism—mortality association was abrogated in those with a body mass index ≤20 kg/m2. In summarizing these collective observational studies, various conclusions may be drawn regarding the association of hypothyroidism with cardiovascular disease and death in CKD (Figure 2).
Figure 2. Potential pathways of the association between thyroid dysfunction and mortality in chronic kidney disease patients.
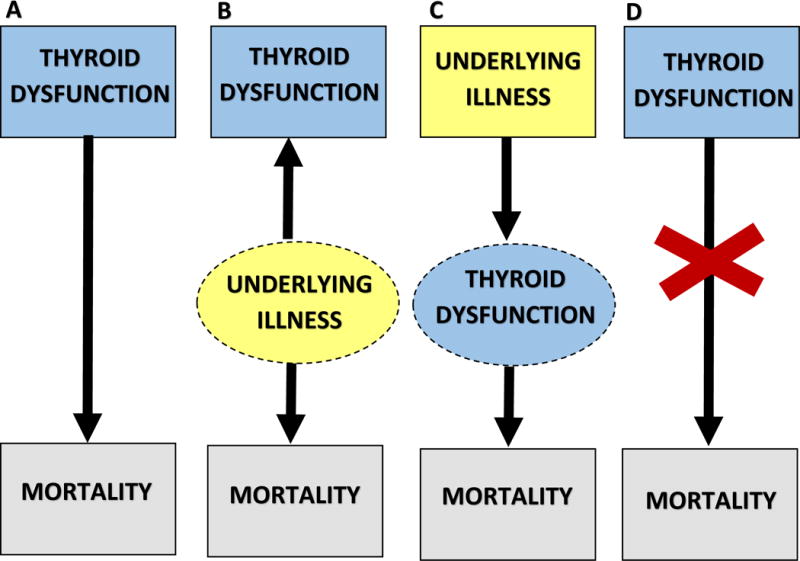
Panel A: Thyroid dysfunction is causally associated with mortality. Panel B: Thyroid dysfunction is a marker of illness, which in and of itself is associated with mortality. Panel C: Thyroid dysfunction is an intermediate pathway between illness and mortality. Panel D: Thyroid dysfunction is not associated with mortality in certain populations (i.e., patients with protein-energy wasting).
Management of Hypothyroidism in Chronic Kidney Disease
Future studies examining the impact of exogenous thyroid hormone treatment upon cardiovascular disease and death may yield key insights into the causal implications of hypothyroidism in CKD. Indeed, the United States Renal Data System Annual Data Report has shown that levothyroxine is among the most commonly prescribed medications in non-dialysis dependent CKD and end-stage renal disease who are Medicare Part D enrollees.[65] However, there has been a paucity of data examining exogenous thyroid hormone replacement in hypothyroid CKD patients. As noted above, observational studies have shown that receipt of exogenous thyroid hormone replacement in patients with subclinical hypothyroidism and CKD was associated with decreased CKD progression.[36, 37, 65] Similarly, in a double-blinded randomized controlled trial of 136 patients with subclinical hypothyroidism (defined as TSH of 4.0–7.0 mIU/L and serum thyroid peroxidase antibody positivity) and early type 2 diabetic nephropathy, treatment with levothyroxine for 48 weeks led to greater reductions in urinary albumin excretion rate, as well as in total and LDL cholesterol, in comparison to placebo.[75] Notably, six patients in the treatment arm experienced adverse cardiovascular reactions including mild paroxysmal supraventricular tachycardia (n=1) and palpitations (n=5). To date, there has been one study that has examined exogenous thyroid hormone replacement and mortality in hypothyroid CKD patients. Among 2715 dialysis patients whose thyroid function and treatment status were ascertained at baseline, those who were euthyroid on treatment (presumed to be “hypothyroid-treated-to-target”) had a similar mortality risk compared to those who were spontaneously euthyroid, whereas patients who were hypothyroid irrespective of treatment status had higher mortality risk.[8]
While these limited data suggest benefit, levothyroxine bears a narrow toxic-to-therapeutic window,[76] and iatrogenic thyrotoxicosis and/or unwarranted treatment may theoretically lead to complications such as increased protein catabolism, reduced bone mineral density, and arrhythmia, particularly in advanced CKD patients with high underlying cardiovascular risk.[1, 5, 77] Thus, at this time, rigorous longitudinal studies and future randomized controlled trials with larger sample sizes and extended follow up periods will provide more definitive information on the safety and effectiveness of exogenous thyroid hormone supplementation in hypothyroid CKD patients.
Conclusion
There are many remaining gaps in knowledge with regards to the interaction between thyroid and kidney disease. Further mechanistic studies are needed to understand the pathogenesis of thyroid functional disorders in kidney disease, as well as how CKD may engender thyroid dysfunction. Future research is also needed to identify more sensitive and specific methods of classifying thyroid functional status in CKD patients, in order to more accurately distinguish true thyroid functional disease from non-thyroidal illness in this population. Given that thyroid hormone receptors are present in nearly all tissues, hypothyroidism may have pervasive effects on multiple end-organs (e.g., neuropsychiatric, hematologic, musculoskeletal); thus, further studies are needed to determine the underlying mechanistic pathways by which hypothyroidism is linked with mortality. Lastly, rigorous studies of exogenous thyroid treatment, including dosing and biochemical targets, and kidney disease progression, cardiovascular disease, and mortality are needed to better understand the causal implications of hypothyroidism in CKD patients.
KEY POINTS.
CKD patients have a disproportionately higher prevalence of hypothyroidism, although many cases may remain latent and undiagnosed.
The thyroid—kidney mechanistic link has not been fully elucidated, but may be bi-directional.
Thyroid functional derangements, including hypothyroidism, are linked with cardiovascular disease and death in CKD patients.
While limited data suggest benefit, future studies are needed to determine the safety and effectiveness of exogenous thyroid hormone replacement in hypothyroid CKD patients.
Acknowledgments
None.
Financial Support and Sponsorship
CMR is supported by a research grant from the NIH/NIDDK (K23-DK102903).
ABBREVATIONS
- CKD
chronic kidney disease
- TSH
thyrotropin
- T4
thyroxine
- eGFR
estimated glomerular filtration rate
- NHANES III
Third National Health and Nutrition Examination Survey
- GBM
glomerular basement membrane
- T3
triiodothyronine
- TRH
thyrotropin-releasing hormone
- 4D study
Deutsche Diabetes Dialyse Studie
Footnotes
Conflicts of Interest
None.
References
- 1*.Rhee CM, Brent GA, Kovesdy CP, et al. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2015;30:724–737. doi: 10.1093/ndt/gfu024. This is a comprehensive review of studies of thyroid functional disease and cardiovascular outcomes in the pre-dialysis and dialysis-dependent CKD populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladenson PW. Diagnosis of Hypothyroidism. In: Braverman LE, Cooper DS, editors. Werner and Ingbar’s The Thyroid. 10th. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 6064–611. [Google Scholar]
- 3.Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney international. 2005;67:10474–1052. doi: 10.1111/j.1523-1755.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocrine reviews. 1996;17:454–63. doi: 10.1210/edrv-17-1-45. [DOI] [PubMed] [Google Scholar]
- 5.Lim VS. Thyroid function in patients with chronic renal failure. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2001;38:S804–84. doi: 10.1053/ajkd.2001.27410. [DOI] [PubMed] [Google Scholar]
- 6.Drechsler C, Schneider A, Gutjahr-Lengsfeld L, et al. Thyroid function, cardiovascular events, and mortality in diabetic hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014;63:9884–996. doi: 10.1053/j.ajkd.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Kang EW, Nam JY, Yoo TH, et al. Clinical implications of subclinical hypothyroidism in continuous ambulatory peritoneal dialysis patients. American journal of nephrology. 2008;28:9084–913. doi: 10.1159/000141933. [DOI] [PubMed] [Google Scholar]
- 8.Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and Mortality among Dialysis Patients. Clinical journal of the American Society of Nephrology: CJASN. 2013;8:5934–601. doi: 10.2215/CJN.06920712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Rhee CM, Kim S, Gillen DL, et al. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. The Journal of clinical endocrinology and metabolism. 2015;100:13864–1395. doi: 10.1210/jc.2014-4311. This is the largest observational study of thyroid function and mortality conducted in dialysis patients to date, showing that hypothyroidism as well as high-normal TSH levels were associated with higher death risk in a national hemodialysis cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Rhee CM, Kalantar-Zadeh K, Streja E, et al. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2015;30:2824–287. doi: 10.1093/ndt/gfu303. This is a rigorous analysis of over 400,000 US veterans showing that impaired kidney function was associated with higher risk of hypothyroidism, independent of socio-demographics and comorbidities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chonchol M, Lippi G, Salvagno G, et al. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN. 2008;3:12964–1300. doi: 10.2215/CJN.00800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng YY, Wu SC, Lin HD, et al. Prevalence of clinical and subclinical thyroid disease in a peritoneal dialysis population. Peritoneal dialysis international: journal of the International Society for Peritoneal Dialysis. 2012;32:864–93. doi: 10.3747/pdi.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee CM, Curhan GC, Alexander EK, et al. Subclinical hypothyroidism and survival: the effects of heart failure and race. The Journal of clinical endocrinology and metabolism. 2013;98:23264–2336. doi: 10.1210/jc.2013-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shantha GP, Kumar AA, Bhise V, et al. Prevalence of Subclinical Hypothyroidism in Patients with End-Stage Renal Disease and the Role of Serum Albumin: A Cross-Sectional Study from South India. Cardiorenal medicine. 2011;1:2554–260. doi: 10.1159/000332757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariani LH, Berns JS. The renal manifestations of thyroid disease. Journal of the American Society of Nephrology: JASN. 2012;23:224–26. doi: 10.1681/ASN.2010070766. [DOI] [PubMed] [Google Scholar]
- 16.Bentley AG, Madsen KM, Davis RG, Tisher CC. Response of the medullary thick ascending limb to hypothyroidism in the rat. The American journal of pathology. 1985;120:2154–221. [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley SE, Coelho JB, Sealey JE, et al. Changes in glomerulotubular dimensions, single nephron glomerular filtration rates and the renin-angiotensin system in hypothyroid rats. Life sciences. 1982;30:6334–639. doi: 10.1016/0024-3205(82)90279-x. [DOI] [PubMed] [Google Scholar]
- 18.Canavan JP, Holt J, Easton J, et al. Thyroid-induced changes in the growth of the liver, kidney, and diaphragm of neonatal rats. Journal of cellular physiology. 1994;161:494–54. doi: 10.1002/jcp.1041610107. [DOI] [PubMed] [Google Scholar]
- 19.Davis RG, Madsen KM, Fregly MJ, Tisher CC. Kidney structure in hypothyroidism. The American journal of pathology. 1983;113:414–49. [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Gomez I, Banegas I, Wangensteen R, et al. Influence of thyroid state on cardiac and renal capillary density and glomerular morphology in rats. The Journal of endocrinology. 2013;216:434–51. doi: 10.1530/JOE-12-0208. [DOI] [PubMed] [Google Scholar]
- 21.Salomon MI, Di Scala V, Grishman E, et al. Renal lesions in hypothyroidism: a study based on kidney biopsies. Metabolism: clinical and experimental. 1967;16:8464–852. doi: 10.1016/0026-0495(67)90186-2. [DOI] [PubMed] [Google Scholar]
- 22.Slotkin TA, Seidler FJ, Kavlock RJ, Bartolome JV. Thyroid hormone differentially regulates cellular development in neonatal rat heart and kidney. Teratology. 1992;45:3034–312. doi: 10.1002/tera.1420450309. [DOI] [PubMed] [Google Scholar]
- 23.Vargas F, Moreno JM, Rodriguez-Gomez I, et al. Vascular and renal function in experimental thyroid disorders. European journal of endocrinology / European Federation of Endocrine Societies. 2006;154:1974–212. doi: 10.1530/eje.1.02093. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley T, Edwards OM. Mild hypothyroidism and oedema: evidence for increased capillary permeability to protein. Clinical endocrinology. 1983;18:6274–635. doi: 10.1111/j.1365-2265.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 25.Asmah BJ, Wan Nazaimoon WM, Norazmi K, et al. Plasma renin and aldosterone in thyroid diseases. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1997;29:5804–583. doi: 10.1055/s-2007-979105. [DOI] [PubMed] [Google Scholar]
- 26.Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian journal of endocrinology and metabolism. 2012;16:2044–213. doi: 10.4103/2230-8210.93737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowley WF, Jr, Ridgway EC, Bough EW, et al. Noninvasive evaluation of cardiac function in hypothyroidism. Response to gradual thyroxine replacement. The New England journal of medicine. 1977;296:14–6. doi: 10.1056/NEJM197701062960101. [DOI] [PubMed] [Google Scholar]
- 28.Singer MA. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2001;37:1644–178. doi: 10.1016/s0272-6386(01)80073-1. [DOI] [PubMed] [Google Scholar]
- 29.Bradley SE, Stephan F, Coelho JB, Reville P. The thyroid and the kidney. Kidney international. 1974;6:3464–365. doi: 10.1038/ki.1974.119. [DOI] [PubMed] [Google Scholar]
- 30.Falk SA, Buric V, Hammond WS, Conger JD. Serial glomerular and tubular dynamics in thyroidectomized rats with remnant kidneys. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1991;17:2184–227. doi: 10.1016/s0272-6386(12)81132-2. [DOI] [PubMed] [Google Scholar]
- 31.Karanikas G, Schutz M, Szabo M, et al. Isotopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. American journal of nephrology. 2004;24:414–45. doi: 10.1159/000075628. [DOI] [PubMed] [Google Scholar]
- 32.Kreisman SH, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Archives of internal medicine. 1999;159:794–82. doi: 10.1001/archinte.159.1.79. [DOI] [PubMed] [Google Scholar]
- 33.Villabona C, Sahun M, Roca M, et al. Blood volumes and renal function in overt and subclinical primary hypothyroidism. The American journal of the medical sciences. 1999;318:2774–280. doi: 10.1097/00000441-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Chang Y, Ryu S, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. International journal of epidemiology. 2014;43:16244–1632. doi: 10.1093/ije/dyu126. [DOI] [PubMed] [Google Scholar]
- 35.Meuwese CL, Gussekloo J, de Craen AJ, et al. Thyroid status and renal function in older persons in the general population. The Journal of clinical endocrinology and metabolism. 2014;99:26894–2696. doi: 10.1210/jc.2013-3778. [DOI] [PubMed] [Google Scholar]
- 36.Shin DH, Lee MJ, Kim SJ, et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. The Journal of clinical endocrinology and metabolism. 2012;97:27324–2740. doi: 10.1210/jc.2012-1663. [DOI] [PubMed] [Google Scholar]
- 37.Shin DH, Lee MJ, Lee HS, et al. Thyroid hormone replacement therapy attenuates the decline of renal function in chronic kidney disease patients with subclinical hypothyroidism. Thyroid: official journal of the American Thyroid Association. 2013;23:6544–661. doi: 10.1089/thy.2012.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Lee SY, Rhee CM, Leung AM, et al. A review: Radiographic iodinated contrast media-induced thyroid dysfunction. The Journal of clinical endocrinology and metabolism. 2015;100:3764–383. doi: 10.1210/jc.2014-3292. This is a review of emerging data on iodinated contrast media as a risk factor for thyroid functional disease in the general and chronic kidney disease populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brough R, Jones C. Iatrogenic iodine as a cause of hypothyroidism in infants with end-stage renal failure. Pediatr Nephrol. 2006;21:4004–402. doi: 10.1007/s00467-005-2115-2. [DOI] [PubMed] [Google Scholar]
- 40.Moisey RS, McPherson S, Wright M, Orme SM. Thyroiditis and iodide mumps following an angioplasty. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2007;22:12504–1252. doi: 10.1093/ndt/gfl777. [DOI] [PubMed] [Google Scholar]
- 41.Sanai T, Inoue T, Okamura K, et al. Reversible primary hypothyroidism in Japanese patients undergoing maintenance hemodialysis. Clinical nephrology. 2008;69:1074–113. doi: 10.5414/cnp69107. [DOI] [PubMed] [Google Scholar]
- 42.Takeda S, Michigishi T, Takazakura E. Iodine-induced hypothyroidism in patients on regular dialysis treatment. Nephron. 1993;65:514–55. doi: 10.1159/000187440. [DOI] [PubMed] [Google Scholar]
- 43.Rhee CM, Lynch KE, Zandi-Nejad K, Pearce EN, Alexander EA, Brunelli SM. Iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism in a community-based cohort. Endocrinology Studies. 2013;3:e8. [Google Scholar]
- 44*.Barr ML, Chiu HK, Li N, et al. Thyroid Dysfunction in Children Exposed to Iodinated Contrast Media. The Journal of clinical endocrinology and metabolism. doi: 10.1210/jc.2016-1330. Epub March 28, 2016. This is the first controlled study conducted to date showing that iodinated contrast media exposure is associated with incident thyroid functional disease in pediatric patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Archives of internal medicine. 2012;172:1534–159. doi: 10.1001/archinternmed.2011.677. [DOI] [PubMed] [Google Scholar]
- 46.Brungger M, Hulter HN, Krapf R. Effect of chronic metabolic acidosis on thyroid hormone homeostasis in humans. The American journal of physiology. 1997;272:F6484–653. doi: 10.1152/ajprenal.1997.272.5.F648. [DOI] [PubMed] [Google Scholar]
- 47.Wiederkehr MR, Kalogiros J, Krapf R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2004;19:11904–1197. doi: 10.1093/ndt/gfh096. [DOI] [PubMed] [Google Scholar]
- 48.Meuwese CL, Dekkers OM, Stenvinkel P, et al. Nonthyroidal illness and the cardiorenal syndrome. Nature reviews. Nephrology. 2013;9:5994–609. doi: 10.1038/nrneph.2013.170. [DOI] [PubMed] [Google Scholar]
- 49.Omrani HR, Rahimi M, Nikseresht K. The effect of selenium supplementation on acute phase reactants and thyroid function tests in hemodialysis patients. Nephrourology monthly. 2015;7:e24781. doi: 10.5812/numonthly.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feinstein EI, Kaptein EM, Nicoloff JT, Massry SG. Thyroid function in patients with nephrotic syndrome and normal renal function. American journal of nephrology. 1982;2:704–76. doi: 10.1159/000166587. [DOI] [PubMed] [Google Scholar]
- 51.Robey C, Shreedhar K, Batuman V. Effects of chronic peritoneal dialysis on thyroid function tests. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1989;13:994–103. doi: 10.1016/s0272-6386(89)80125-8. [DOI] [PubMed] [Google Scholar]
- 52.Carrero JJ, Stenvinkel P, Lindholm B. Endocrine Aspects of Chronic Kidney Disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, editors. Taal: Brenner and Rector’s The Kidney. 9th. Philadelphia: Elsevier Saunders; 2012. pp. 21224–2137. [Google Scholar]
- 53.Wiersinga WM, Van den Berghe G. Nonthyroidal Illness Syndrome. In: Braverman LE, Cooper DS, editors. Werner and Ingbar’s The Thyroid. 10th. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 2034–216. [Google Scholar]
- 54.Song SH, Kwak IS, Lee DW, et al. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2009;24:15344–1538. doi: 10.1093/ndt/gfn682. [DOI] [PubMed] [Google Scholar]
- 55.Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine reviews. 2002;23:384–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 56.Langton JE, Brent GA. Nonthyroidal illness syndrome: evaluation of thyroid function in sick patients. Endocrinology and metabolism clinics of North America. 2002;31:1594–172. doi: 10.1016/s0889-8529(01)00008-1. [DOI] [PubMed] [Google Scholar]
- 57.Soldin OP. Measuring serum thyroid-stimulating hormone, thyroid hormones, thyroid-directed antibodies, and transport proteins. In: Braverman LE, Cooper DS, editors. Werner and Ingbar’s The Thyroid. 10th. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 2794–297. [Google Scholar]
- 58.Soldin OP, Soldin SJ. Thyroid hormone testing by tandem mass spectrometry. Clinical biochemistry. 2011;44:894–94. doi: 10.1016/j.clinbiochem.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soldin SJ, Soukhova N, Janicic N, et al. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clinica chimica acta; international journal of clinical chemistry. 2005;358:1134–118. doi: 10.1016/j.cccn.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clinical chemistry. 2009;55:13804–1388. doi: 10.1373/clinchem.2008.118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jonklaas J, Soldin SJ. Tandem mass spectrometry as a novel tool for elucidating pituitary-thyroid relationships. Thyroid: official journal of the American Thyroid Association. 2008;18:13034–1311. doi: 10.1089/thy.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clinical chemistry. 2011;57:1224–127. doi: 10.1373/clinchem.2010.154088. [DOI] [PubMed] [Google Scholar]
- 63.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. The New England journal of medicine. 2001;344:5014–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 64.Wheeler DC, Haynes R, Landray MJ, Baigent C. Cardiovascular Aspects of Kidney Disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, editors. Taal: Brenner and Rector’s The Kidney. 9th. Philadelphia: Elsevier Saunders; 2012. pp. 20604–2075. [Google Scholar]
- 65.United States Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: 2014. [Google Scholar]
- 66.Jaroszynski AJ, Glowniak A, Chrapko B, et al. Low-T3 syndrome and signal-averaged ECG in haemodialysed patients. Physiological research / Academia Scientiarum Bohemoslovaca. 2005;54:5214–526. [PubMed] [Google Scholar]
- 67.Meuwese CL, Carrero JJ, Cabezas-Rodriguez I, et al. Nonthyroidal illness: a risk factor for coronary calcification and arterial stiffness in patients undergoing peritoneal dialysis? Journal of internal medicine. 2013;274:5844–593. doi: 10.1111/joim.12107. [DOI] [PubMed] [Google Scholar]
- 68**.Meuwese CL, Olauson H, Qureshi AR, et al. Associations between Thyroid Hormones, Calcification Inhibitor Levels and Vascular Calcification in End-Stage Renal Disease. PloS one. 2015;10:e0132353. doi: 10.1371/journal.pone.0132353. This is a rigorous study demonstrating that lower FT3, FT4, and TSH levels are inversely associated with coronary calcification in end-stage renal disease patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tatar E, Kircelli F, Asci G, et al. Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clinical journal of the American Society of Nephrology: CJASN. 2011;6:22404–2246. doi: 10.2215/CJN.02540311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tatar E, Sezis Demirci M, Kircelli F, et al. The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. International urology and nephrology. 2012;44:6014–606. doi: 10.1007/s11255-011-0034-7. [DOI] [PubMed] [Google Scholar]
- 71.Yilmaz MI, Sonmez A, Karaman M, et al. Low triiodothyronine alters flow-mediated vasodilatation in advanced nondiabetic kidney disease. American journal of nephrology. 2011;33:254–32. doi: 10.1159/000322581. [DOI] [PubMed] [Google Scholar]
- 72.Zoccali C, Benedetto F, Mallamaci F, et al. Low triiodothyronine and cardiomyopathy in patients with end-stage renal disease. Journal of hypertension. 2006;24:20394–2046. doi: 10.1097/01.hjh.0000244954.62362.8f. [DOI] [PubMed] [Google Scholar]
- 73.Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nature reviews. Endocrinology. 2012;8:4174–424. doi: 10.1038/nrendo.2012.29. [DOI] [PubMed] [Google Scholar]
- 74.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA: the journal of the American Medical Association. 2010;304:13654–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75**.Liu P, Liu R, Chen X, et al. Can levothyroxine treatment reduce urinary albumin excretion rate in patients with early type 2 diabetic nephropathy and subclinical hypothyroidism? A randomized double-blind and placebo-controlled study. Current medical research and opinion. 2015;31:22334–2240. doi: 10.1185/03007995.2015.1094044. This is a double-blind randomized controlled trial showing the levothyroxine administration improved urinary albumin excretion rate and lipid profiles in patients with subclinical hypothyroidism an early type 2 diabetic nephropathy. [DOI] [PubMed] [Google Scholar]
- 76.Jonklaas J. Treatment of Hypothyroidism. In: Braverman LE, Cooper DS, editors. Werner and Ingbar’s The Thyroid. 10th. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 6114–627. [Google Scholar]
- 77*.Mammen JS, McGready J, Oxman R, et al. Thyroid Hormone Therapy and Risk of Thyrotoxicosis in Community-Resident Older Adults: Findings from the Baltimore Longitudinal Study of Aging. Thyroid: official journal of the American Thyroid Association. 2015;25:9794–986. doi: 10.1089/thy.2015.0180. This study demonstrated that a large proportion of low TSH levels were due to iatrogenic thyrotoxicosis in a community based cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]


