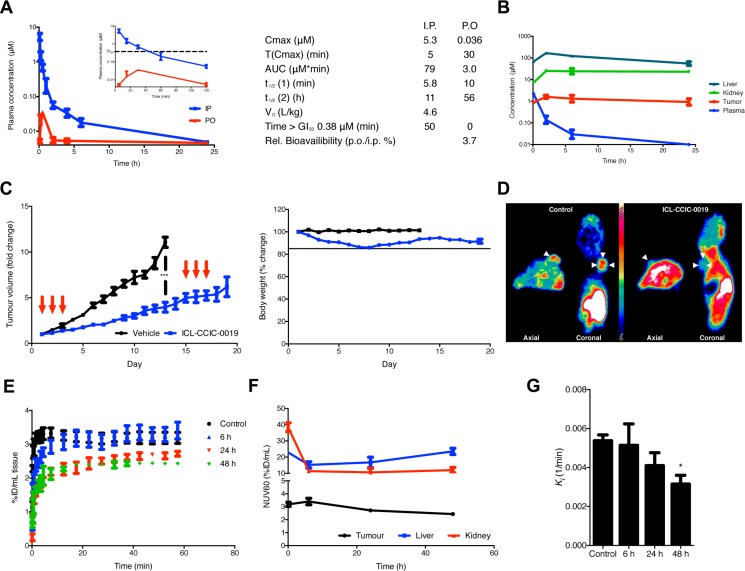
Figure 4. Plasma and tissue pharmacokinetics of ICL-CCIC-0019.
(A) BALB/c mice were administered 10 mg/kg ICL-CCIC-0019 p.o. or i.p. and plasma obtained 5, 15, 30, 60 minutes and 2, 4, 6 and 24 hours post injection. Figure insert displays first 120 minutes only. Plasma pharmacokinetic variables are shown. (B) Tissue distribution of ICL-CCIC-0019. HCT116 xenograft bearing BALB/c nude mice were treated with 10 mg/kg ICL-CCIC-0019 and plasma, tumor, liver and kidney inhibitor concentrations determined at indicated time points. (C) Antitumor activity of ICL-CCIC-0019 and body weight changes in a HCT116 xenograft model. Arrows indicate time of dosing. (D) Representative PET image using the target-competitive probe [18F]-D4-FCH in ICL-CCIC-0019-treated HCT116 xenografts. (E) TACs of dynamic PET imaging scans. (F) Tumor, liver and kidney-associated radioactivity upon ICL-CCIC-0019 treatment. (G) The pharmacokinetic macro rate constant for net irreversible uptake, Ki, was computed employing a two-tissue irreversible compartmental model. Data represent mean of n = 4–5 per group ± SEM; *P ≤ 0.05.