Figure 3. PKCδ phosphorylates E-cadherin at Thr790 in vitro and in intact cells.
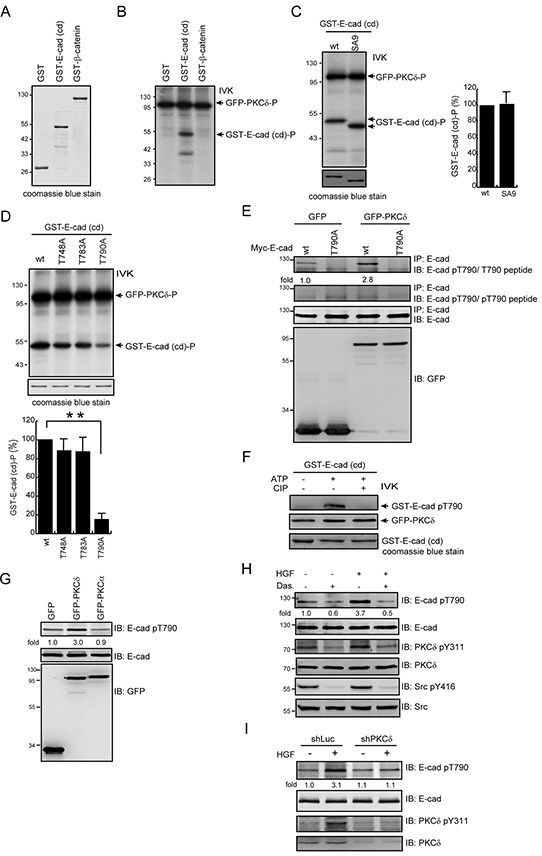
A. GST, GST-E-cadherin-cytoplasmic domain (cd), and GST-β-catenin were purified, fractionated by SDS-polyacrylamide gel electrophoresis, and visualized by Coomassie blue staining. B. GFP-PKCδ was transiently expressed in HEK293 cells and was then immunoprecipitated using an anti-GFP antibody. The immunocomplexes were subjected to an in vitro kinase assay using GST, GST-E-cadherin-cd, or GST-β-catenin as a substrate. The 32P-incorporated proteins were fractionated by SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. IVK, in vitro kinase assay. C. GFP-PKCδ was immunoprecipitated by anti-GFP antibody and the immunocomplexes were subjected to an in vitro kinase assay using purified GST-E-cadherin-cd or its SA9 mutant as a substrate. The SA9 mutant consists of nine mutations at the serine resides (S829, S838, S840, S844, S846, S847, S850, S851, and S853) in the highly conserved serine cluster region (a.a. 840-855) of the classical E-cadherins. The radioisotope activity of the 32P-incorporated GST-E-cadherin-cd proteins was measured and expressed as the percentage relative to the level of the GST-E-cadherin-cd wt. The values (mean ± SD) are from three experiments. D. The GST-E-cadherin-cd or its mutants T748A, T783A, and T790A were purified and served as a substrate for GFP-PKCδ in vitro. The 32P-incorporated proteins were fractionated by SDS-polyacrylamide gel electrophoresis and visualized by autoradiography. The radioisotope activity of the 32P-incorporated GST-E-cadherin-cd proteins was measured and expressed as the percentage relative to the level of the GST-E-cadherin-cd wt. The values (mean ± SD) are from three experiments. **, P < 0.01. E. Myc-tagged E-cadherin (Myc-E-cadherin) was transiently co-expressed with GFP or GFP-PKCδ in CHO cells. Myc-E-cadherin was immunoprecipitated by anti-E-cadherin antibody and the immunocomplexes were analyzed by immunoblotting (IB) with anti-E-cad pT790 antibody in the presence of pT790 peptides or T790 peptides. The level of Myc-E-cadherin pT790 was quantified and expressed as fold relative to the level in the cells co-expressing GFP and Myc-E-cadherin wt. F. Purified GST-E-cadherin-cd proteins were phosphorylated by GFP-PKCδ in the presence of 1mM ATP and dephosphorylated by CIP in vitro. The phosphorylated and dephosphorylated GST-E-cadherin-cd proteins were analyzed by immunoblotting with anti-E-cad pT790 antibody. G. MDCK cells stably expressing GFP, GFP-PKCδ or GFP-PKCα were grown to confluence and then lysed. The cell lysates were analyzed by immunoblotting with antibodies to E-cadherin and E-cadherin pT790. The level of E-cadherin pT790 was quantified and expressed as fold relative to the level in the cells expressing GFP. H. MDCK cells were serum-starved for 24 h and were then treated with (+) or without (−) dasatinib (100 nM) and/or HGF (20 ng/ml) for 15 min. To detect E-cadherin pT790, E-cadherin was immunoprecipitated by anti-E-cadherin antibody (clone 36) and the immunocomplexes were analyzed by immunoblotting with antibodies to E-cadherin and E-cadherin pT790. The level of E-cadherin pT790 was quantified and expressed as fold relative to the level of the control. I. MDCK cells stably expressing shRNA specific to canine PKCδ were serum-starved for 24 h and then treated with or without HGF (20 ng/ml) for 15 min. The cell lysates were analyzed by immunoblotting with the indicated antibodies. The level of E-cadherin pT790 was quantified and expressed as fold relative to the level of the cells expressing shPKCδ without HGF treatment.
