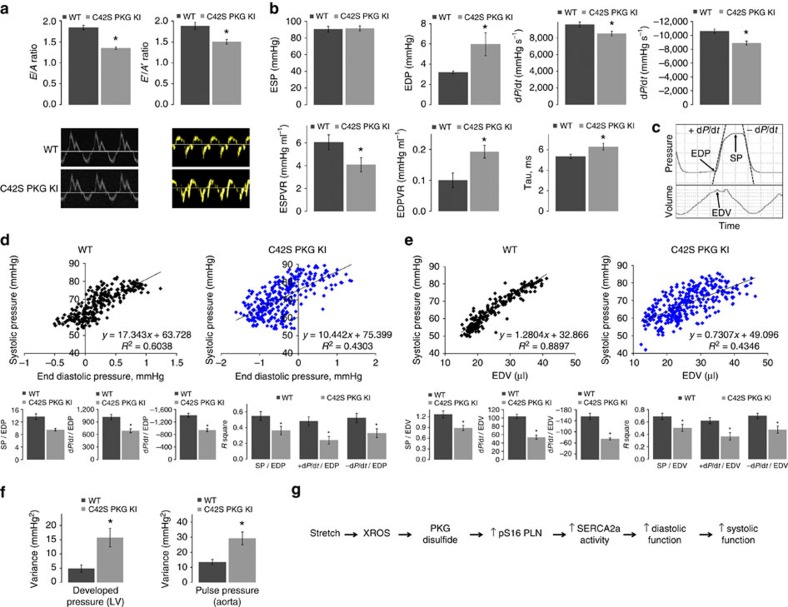
Figure 4. Impaired diastolic relaxation and Frank–Starling mechanism in C42S PKGIα KI mice in vivo.
(a) E/A and E′/A′ ratios for WT and KI mice measured by tissue Doppler echocardiography. Both ratios are significantly decreased in the mutant indicating diastolic dysfunction (*P<0.05; n=5). (b) Various cardiac parameters for the WT and KI mice derived from left ventricular PV loops. EDP, end-diastolic pressure-volume relationship and Tau were significantly increased in the KI, while the rates of relaxation and contraction and end-systolic pressure-volume relationship were significantly decreased (*P<0.05; n=9–10 for baseline measurements except inferior vena cava where n=7–8). These observations are indicative of reduced contractility and impaired myocardial relaxation in the KI. (c) Representative trace of pressure and volume measured in the left ventricle of a WT or KI mouse over the time period of one cardiac cycle. EDP, SP, EDV, +dp/dt and −dp/dt were determined as indicated so that intra-beat relationships could be calculated that related directly to the Frank–Starling mechanism in vivo. (d) Representative scatter plot of SP versus EDP for a WT and KI mouse; data was obtained from 200 heartbeats as described above. Histograms show the averaged gradients for SP, +dp/dt and −dp/dt versus EDP and the corresponding mean coefficients of determination, R2. Intra-beat relationships, as well as R2 values were significantly decreased for each variable in the KI hearts (*P<0.05; n=9). (e) A representative scatter plot of SP versus EDV (an index of myocardial stretch) for a WT and KI mouse. Intra-beat relationships and associated R2 values were determined as described above for EDP and, similarly, were significantly decreased for the KI in each case (*P<0.05; n=9). These results provide compelling evidence that the PKGIα Cys42 disulfide bond contributes to the Frank–Starling mechanism. (f) Variance in the LV developed pressure (SP−EDP; n=9) and aortic pulse pressure (n=7–10) in 1000 consecutive heartbeats for WT or KI mice. Both pressures were significantly more variable in the KI mice (*P<0.05) further demonstrating dysregulation of cardiac output when PKGIα cannot be oxidant-activated. (g) Scheme showing how oxidation of PKGIα by stretch-induced oxidants contributes to the Frank–Starling response. Error bars show s.e.m. P values were determined by t-test.