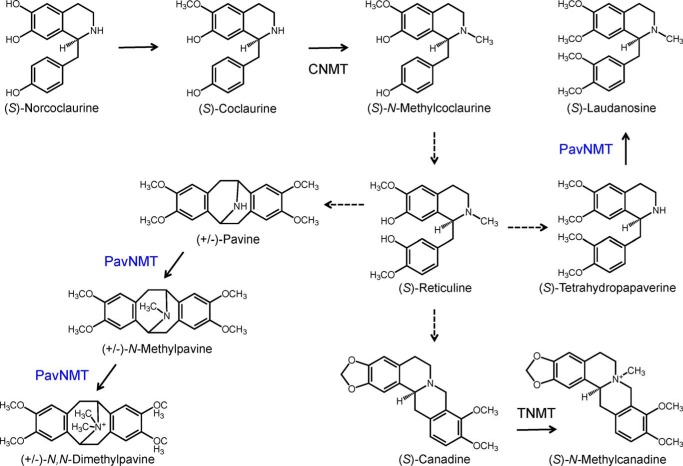
FIGURE 1.
Reactions catalyzed by NMTs in BIA biosynthesis. CNMT is mainly responsible for the N-methylation of the 1-benzylisoquinonline (S)-coclaurine, generating the tertiary amine in the central branch point intermediate (S)-reticuline. In contrast, TNMT primarily accepts a variety of protoberberine alkaloids, such as (S)-canadine, yielding quaternary derivatives. PavNMT is known to prefer substrates with a pavine scaffold compared with those containing 1-benzylisoquinonline or protoberberine backbones.