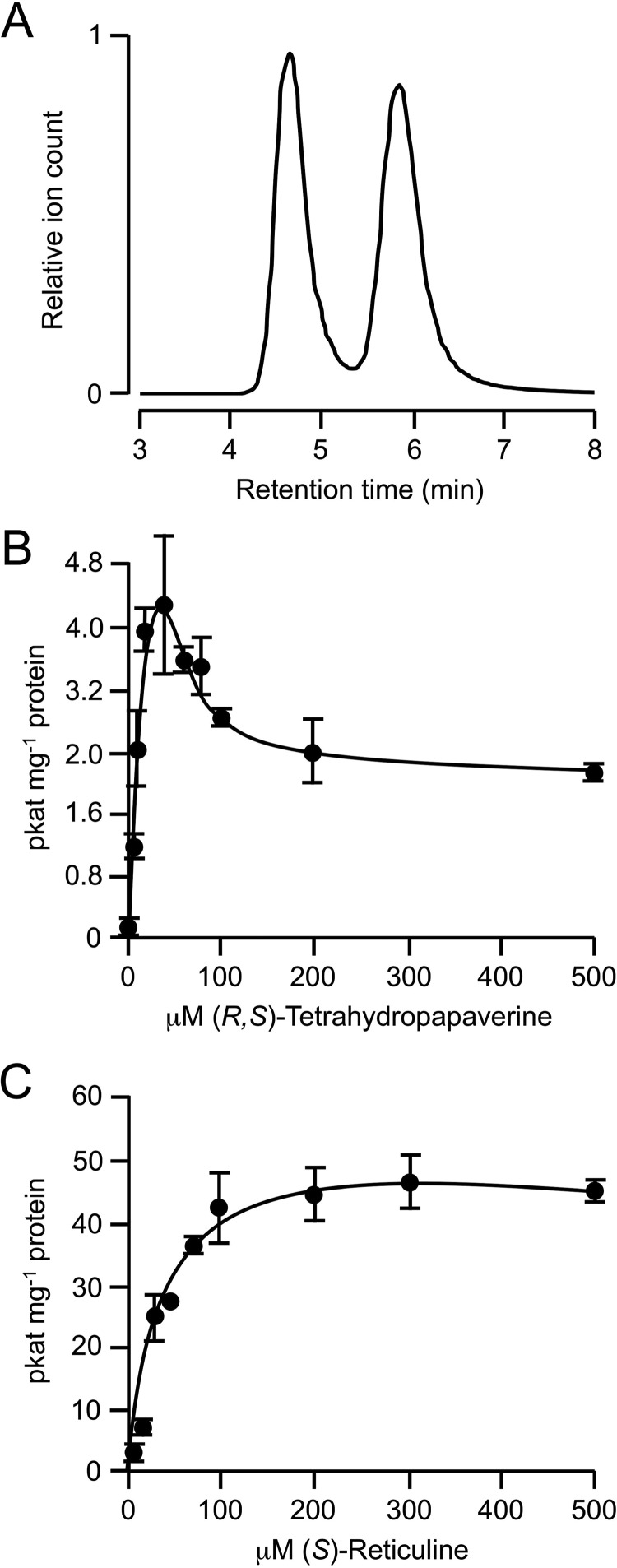
FIGURE 2.
Kinetic analysis of PavNMT using racemic THP as the enzyme substrate. A, separation of R and S enantiomers of THP using chiral chromatography. B, plot of initial reaction velocity of PavNMT versus the concentration of a racemic mixture of THP as the enzyme substrate. Values represent the mean ± S.D. (error bars) of three independent assays, and the data were fit to a rate equation derived using rapid equilibrium assumptions for the formation of productive E·S and non-productive E·S·S complexes, as expected for substrate inhibition. C, plot of initial reaction velocity of PavNMT versus the concentration of (S)-reticuline as the enzyme substrate.