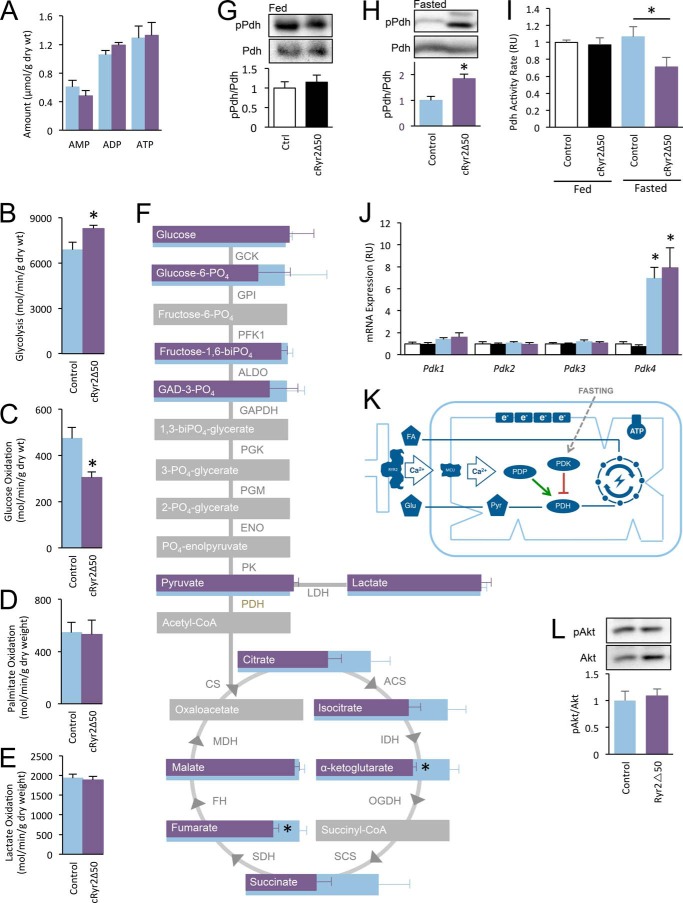
FIGURE 4.
cRyr2Δ50 mice have a specific defect in glucose oxidation, associated with hyperphosphorylation and reduced activity of Pdh. A, average heart ATP, ADP, and AMP as measured by HPLC-chromatography on whole homogenized hearts (n = 3). B and C, non-oxidative glycolysis (B) and complete glucose oxidation (C) were simultaneously measured in working hearts (control n = 5, cRyr2Δ50 n = 6; *, p ≤ 0.05). Light blue bars = control, purple bars = cRyr2Δ50 throughout. D and E, at the same time, palmitate oxidation (D) and lactate oxidation (E) were measured (control n = 5, cRyr2Δ50 n = 7). F, diagram showing the results of targeted mass spectroscopy-based metabolomic analysis of TCA cycle acid intermediaries and glycolysis pathway sugar intermediates (n = 8; *, p ≤ 0.05; gray = unmeasured). GCK, germinal center kinase; GPI, glycosylphosphatidylinositol; PFK1, phosphofructokinase 1; ALDO, aldo-keto reductase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; ENO, eno/pyruvate carboxylase; PK, protein kinase. G and H, pyruvate dehydrogenase kinase E1-α subunit serine 293 phosphorylation levels in mice fed ad libitum (white, control n = 3; black, cRyr2Δ50 n = 3; *, p ≤ 0.05) and fasted overnight (blue, control n = 5; purple, cRyr2Δ50 = 6; *, p ≤ 0.05). pPdh, phospho-Pdh; Ctrl, control. I, pyruvate dehydrogenase activity in ad libitum fed and overnight fasted whole homogenized hearts (fed control n = 7, fed cRyr2Δ50 n = 8, fasted control n = 8, fasted cRyr2Δ50 n = 8; *, p ≤ 0.05). J, pyruvate dehydrogenase kinase isoform mRNA levels in ad libitum fed and fasted cRyr2Δ50 hearts (n = 6, *, p ≤ 0.05). K, working model of context-dependent regulation of PDH activity by mitochondrial Ca2+. L, assessment of Akt phosphorylation levels in overnight fasted cRyr2Δ50 hearts (n = 6; *, p ≤ 0.05). All data were plotted as mean ± S.E.