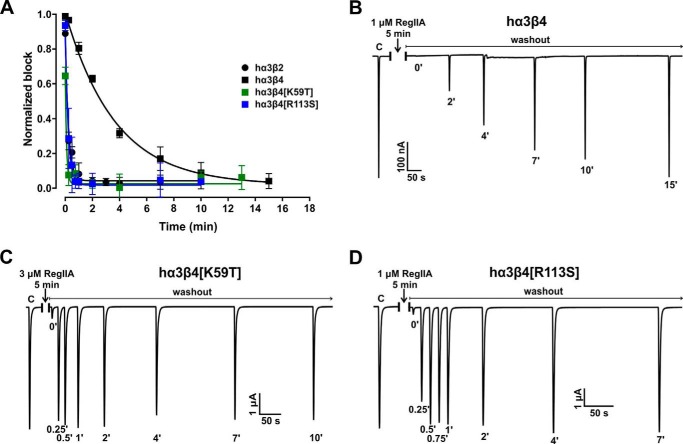
FIGURE 4.
Single residue β4 mutations in hα3β4 increase the α-conotoxin off-rate of RegIIA to values similar to wild-type hα3β2. A, graph summarizing the wash-off kinetics data. Wild-type hα3β2 and hα3β4 data are the same as described in the legend to Fig. 3 and shown for comparison. Replacing loop D residue 59 in hβ4 with the respective residue in hβ2 (mutant hα3β4[K59T], green squares) is sufficient to shift the off-rate to the opposite subtype. A mutant in which residue Arg113 of hβ4 is replaced with Ser, as in hβ2, (hα3β4[R113S], blue squares) similarly exhibits a fast wash-off rate resembling hα3β2. Representative ACh-evoked currents of hα3β4 (B), hα3β4[K59T] (C), and hα3β4[R113S] (D) illustrate the recovery from the RegIIA block differs between wild-type and the two mutant hα3β4 nAChR subtypes. Experimental conditions were the same as those described in the legend to Fig. 3. All data points in A represent mean ± 95% CI, n = 3–7. The times required to reach 95% recovery from block are summarized in Table 3.