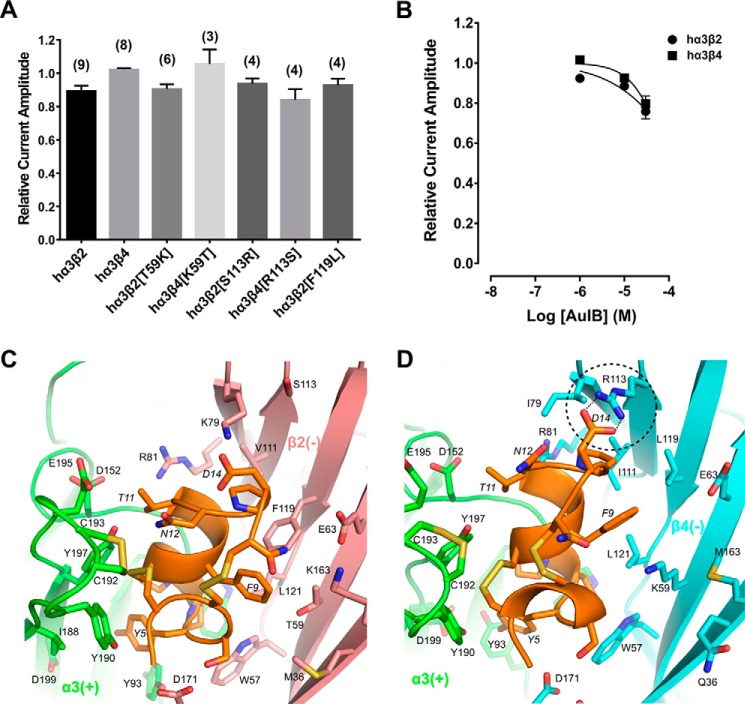
FIGURE 7.
α-Conotoxin AuIB has minor activity at human α3β2 and α3β4 nAChRs. A, bar graph representing potency of block by α-conotoxin AuIB (1 μm) at wild-type and mutant human α3β2 and α3β4 nAChRs. Data represent mean ± S.E., n = 3–9. B, concentration-response analysis of AuIB at wild-type α3β2 and α3β4 nAChRs indicated the IC50 is considerably higher than 30 μm at both nAChR subtypes. AuIB (30 μm) reduced ACh-evoked current amplitude mediated by α3β2 to 75.9 ± 3.7% of control (n = 10) and α3β4 currents to 79.6 ± 4.0% (n = 10), respectively. C and D, molecular dynamics simulation predicted binding modes of AuIB to α3β2 (C) and α3β4 (D). Several hydrogen bonds are formed between pairwise interacting residues of different loops and between toxin and receptor, e.g. AuIB Asp14 with β4 Arg113 (dashed circle, hydrogen bonds as dotted lines). The α3(+) interface is shown in green, β2(−) in pink, β4(−) in cyan, and AuIB in orange. Non-conserved residues are shown as licorice models and labeled. Residues from the receptor and AuIB are labeled using normal and italic fonts, respectively.